| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The Gram-negative porin 1 (GBP-1) Family [Function: Non-specific diffusion channels] Seed alignment | Full alignment | Pfam page | Pfam Wiki page | TC-DB page | ||||
This family consists of the general diffusion porins from mainly the Beta and Gamma subdivisions of Proteobacteria. Although the porins of this family share common structural and functional properties with the porins of General Bacterial Porin Family 2 (GBP-2) and the Rhodobacter porin family, their sequence similarity is rather low. The porins form large aqueous channels, facilitating the diffusion of small hydrophilic molecules through the outer membrane in a non-specific manner. Important aspects of the channel activity are the conductance, the voltage dependence and the pH sensitivity. Several porins of this family have been determined at low resolution (Omp32, OmpF, Phosphoporin, OmpK36) suggesting that the 16-stranded ß-barrel is a common structural feature. In addition to that, the porins of this family seem to require trimerization in order to function properly. In Providencia stuartii, these porins form primitive junctions between P. stuartii cells. These junctions could foster the formation of floating communities and support intercellular communications. | ||||
Representative image: 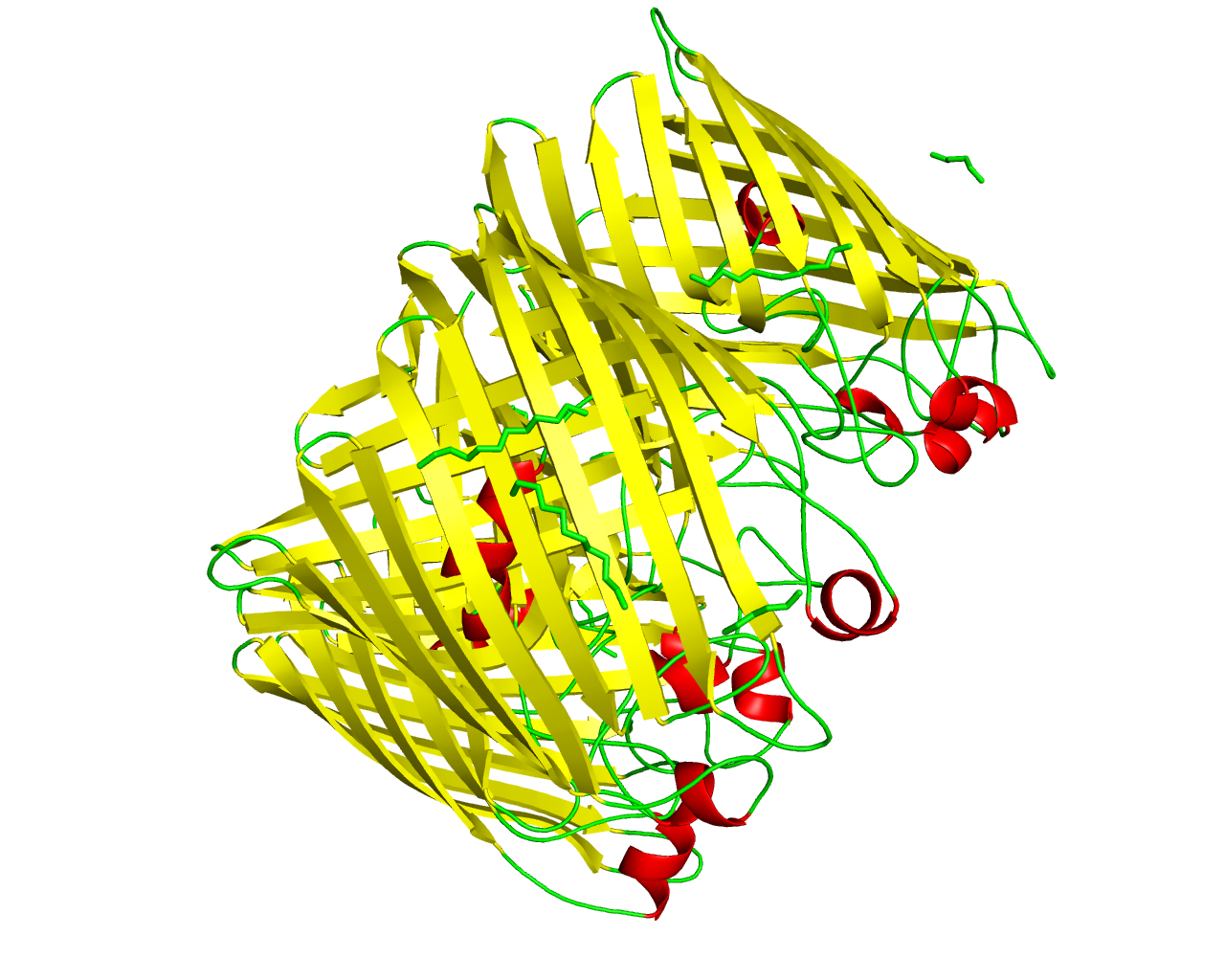 | ||||
| Literature references | ||||
Outer Membrane Proteins OmpA, FhuA, OmpF, EstA, BtuB, and OmpX Have Unique Lipopolysaccharide Fingerprints J Chem Theory Comput. 2019 Apr 9;15(4):2608-2619. doi: 10.1021/acs.jctc.8b01059. Epub 2019 Mar 21. PMID: 30848905 | ||||
Lipid Headgroup Charge and Acyl Chain Composition Modulate Closure of Bacterial ß-Barrel Channels Int J Mol Sci. 2019 Feb 5;20(3):674. doi: 10.3390/ijms20030674. PMID: 30764475 | ||||
Getting Drugs into Gram-Negative Bacteria: Rational Rules for Permeation through General Porins ACS Infect Dis. 2018 Oct 12;4(10):1487-1498. doi: 10.1021/acsinfecdis.8b00108. Epub 2018 Aug 17. PMID: 29962203 | ||||
Porin self-association enables cell-to-cell contact in Providencia stuartii floating communities Proc Natl Acad Sci U S A. 2018 Mar 6;115(10):E2220-E2228. doi: 10.1073/pnas.1714582115. Epub 2018 Feb 23. PMID: 29476011 | ||||
Influence of the surrounding environment in re-naturalized ß-barrel membrane proteins Biophys Chem. 2018 Mar;234:6-15. doi: 10.1016/j.bpc.2017.12.003. Epub 2018 Jan 2. PMID: 29306652 | ||||
A New Strain Collection for Improved Expression of Outer Membrane Proteins Front Cell Infect Microbiol. 2017 Nov 7;7:464. doi: 10.3389/fcimb.2017.00464. eCollection 2017. PMID: 29164072 | ||||
Engineering a Novel Porin OmpGF Via Strand Replacement from Computational Analysis of Sequence Motif Biochim Biophys Acta Biomembr. 2017 Jul;1859(7):1180-1189. doi: 10.1016/j.bbamem.2017.03.012. Epub 2017 Mar 21. PMID: 28341438 | ||||
Deducing the symmetry of helical assemblies: Applications to membrane proteins J Struct Biol. 2016 Aug;195(2):167-178. doi: 10.1016/j.jsb.2016.05.011. Epub 2016 May 30. PMID: 27255388 | ||||
Magnetically Directed Two-Dimensional Crystallization of OmpF Membrane Proteins in Block Copolymers J Am Chem Soc. 2016 Jan 13;138(1):28-31. doi: 10.1021/jacs.5b03320. Epub 2015 Dec 24. PMID: 26677866 | ||||
Molecular dynamics simulations of membrane proteins under asymmetric ionic concentrations J Gen Physiol. 2013 Oct;142(4):465-75. doi: 10.1085/jgp.201311014. PMID: 24081985 | ||||
Intrinsically disordered protein threads through the bacterial outer-membrane porin OmpF Science. 2013 Jun 28;340(6140):1570-4. doi: 10.1126/science.1237864. PMID: 23812713 | ||||
Ion-channels: goals for function-oriented synthesis Acc Chem Res. 2013 Dec 17;46(12):2773-80. doi: 10.1021/ar400007w. Epub 2013 May 7. PMID: 23651489 | ||||
Asymmetric pore occupancy in crystal structure of OmpF porin from Salmonella typhi J Struct Biol. 2012 Jun;178(3):233-44. doi: 10.1016/j.jsb.2012.04.005. Epub 2012 Apr 16. PMID: 22525817 | ||||
Structural basis for solute transport, nucleotide regulation, and immunological recognition of Neisseria meningitidis PorB Proc Natl Acad Sci U S A. 2010 Apr 13;107(15):6811-6. doi: 10.1073/pnas.0912115107. Epub 2010 Mar 29. PMID: 20351243 | ||||
Cation-selective pathway of OmpF porin revealed by anomalous X-ray diffraction J Mol Biol. 2010 Feb 19;396(2):293-300. doi: 10.1016/j.jmb.2009.11.042. Epub 2009 Nov 20. PMID: 19932117 | ||||
Crystal structures of the OmpF porin: function in a colicin translocon EMBO J. 2008 Aug 6;27(15):2171-80. doi: 10.1038/emboj.2008.137. Epub 2008 Jul 17. PMID: 18636093 | ||||
Crystal structure of osmoporin OmpC from E. coli at 2.0 A J Mol Biol. 2006 Oct 6;362(5):933-42. doi: 10.1016/j.jmb.2006.08.002. Epub 2006 Aug 3. PMID: 16949612 | ||||
High resolution crystal structures and molecular dynamics studies reveal substrate binding in the porin Omp32 J Biol Chem. 2006 Mar 17;281(11):7413-20. doi: 10.1074/jbc.M510939200. Epub 2006 Jan 23. PMID: 16434398 | ||||
Solute uptake through general porins Front Biosci. 2003 May 1;8:d1055-71. doi: 10.2741/1132. PMID: 12700124 | ||||
Crystal structure of Omp32, the anion-selective porin from Comamonas acidovorans, in complex with a periplasmic peptide at 2.1 A resolution Structure. 2000 Sep 15;8(9):981-92. doi: 10.1016/s0969-2126(00)00189-1. PMID: 10986465 | ||||
Crystal structure and functional characterization of OmpK36, the osmoporin of Klebsiella pneumoniae Structure. 1999 Apr 15;7(4):425-34. doi: 10.1016/s0969-2126(99)80055-0. PMID: 10196126 | ||||
The structure of OmpF porin in a tetragonal crystal form Structure. 1995 Oct 15;3(10):1041-50. doi: 10.1016/s0969-2126(01)00240-4. PMID: 8589999 | ||||
Crystal structures explain functional properties of two E. coli porins Nature. 1992 Aug 27;358(6389):727-33. doi: 10.1038/358727a0. PMID: 1380671 | ||||
The bacterial porin superfamily: sequence alignment and structure prediction Mol Microbiol. 1991 Sep;5(9):2153-64. doi: 10.1111/j.1365-2958.1991.tb02145.x. PMID: 1662760 | ||||
| Proteins in this family with 3D-structure | ||||
View Entry | Description | Organism | Length | # strands |
