| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The Thermus thermophilus HB27 TtoA Family [Function: Unknown] Seed alignment | Full alignment | ||||
The thermophilic eubacterium Thermus thermophilus has a multilayered cell envelope that differs from that of other Gram-negative bacteria. The outer membrane contains integral outer membrane proteins (OMPs), of which only a few are characterized. The structure of TtoA was determined to a resolution of 2.8 A, representing the first crystal structure of an OMP from a thermophilic bacterium. TtoA consists of an 8-stranded ß-barrel with a large extracellular part to which a divalent cation is bound. A 5-stranded extracellular ß sheet protrudes out of the membrane-embedded transmembrane barrel and is stabilized by a disulfide bridge. In other Gram-negative bacteria, the C-terminal signature sequence of OMPs is required for binding to a protein of the Omp85 family as a prerequisite for its assembly and integration into the membrane. There is evidence that a similar assembly pathway exists in T. thermophilus by an in vitro binding assay, where the unfolded TtoA binds to the Thermus Omp85 family protein TtOmp85, while a mutant without the signature sequence does not. Similar proteins were found in other thermophilic bacteria. | ||||
Representative image: 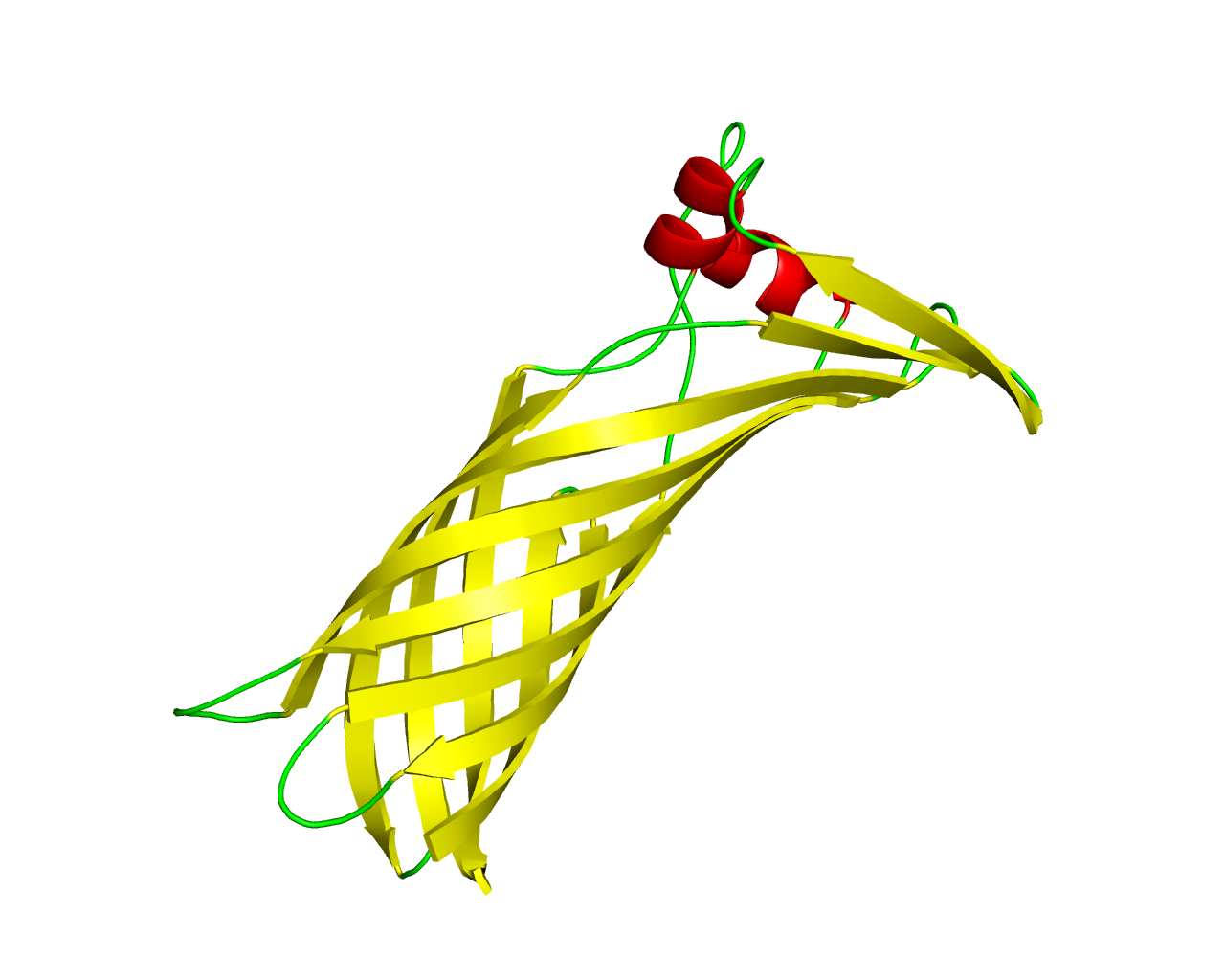 | ||||
| Literature references | ||||
| Proteins in this family with 3D-structure | ||||
View Entry | Description | Organism | Length | # strands |
