| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The Putative cyclodextrin porin (CymA) Family [Function: Specific diffusion channels] Seed alignment | Full alignment | Pfam page | TC-DB page | ||||
CymA of Klebsiella oxytoca, is a porin essential for growth on cyclodextrins, but it can also complement the deficiency of a LamB (maltoporin) mutant of Escherichia coli for growth on linear maltodextrins, indicating that both cyclic and linear oligosaccharides are accepted as substrates. This protein forms ion-permeable channels, which exhibit a substantial current noise. CymA-induced membrane conductance decreased considerably upon addition of alpha-cyclodextrin, whereas the affinity was lower for ß-cyclodextrin and even lower for gamma-cyclodextrin. Unlike most bacterial porins, cymA does not form trimeric complexes in lipid membranes and shows no tendency to trimerise in solution. However, it seems to form homotetramers with a central pore, and therefore lacks the typical trimeric structure of most porins. It is also speculated it be distantly related to OmpG. A recently published structure shows that members of this family have 14 TM segments. | ||||
Representative image: 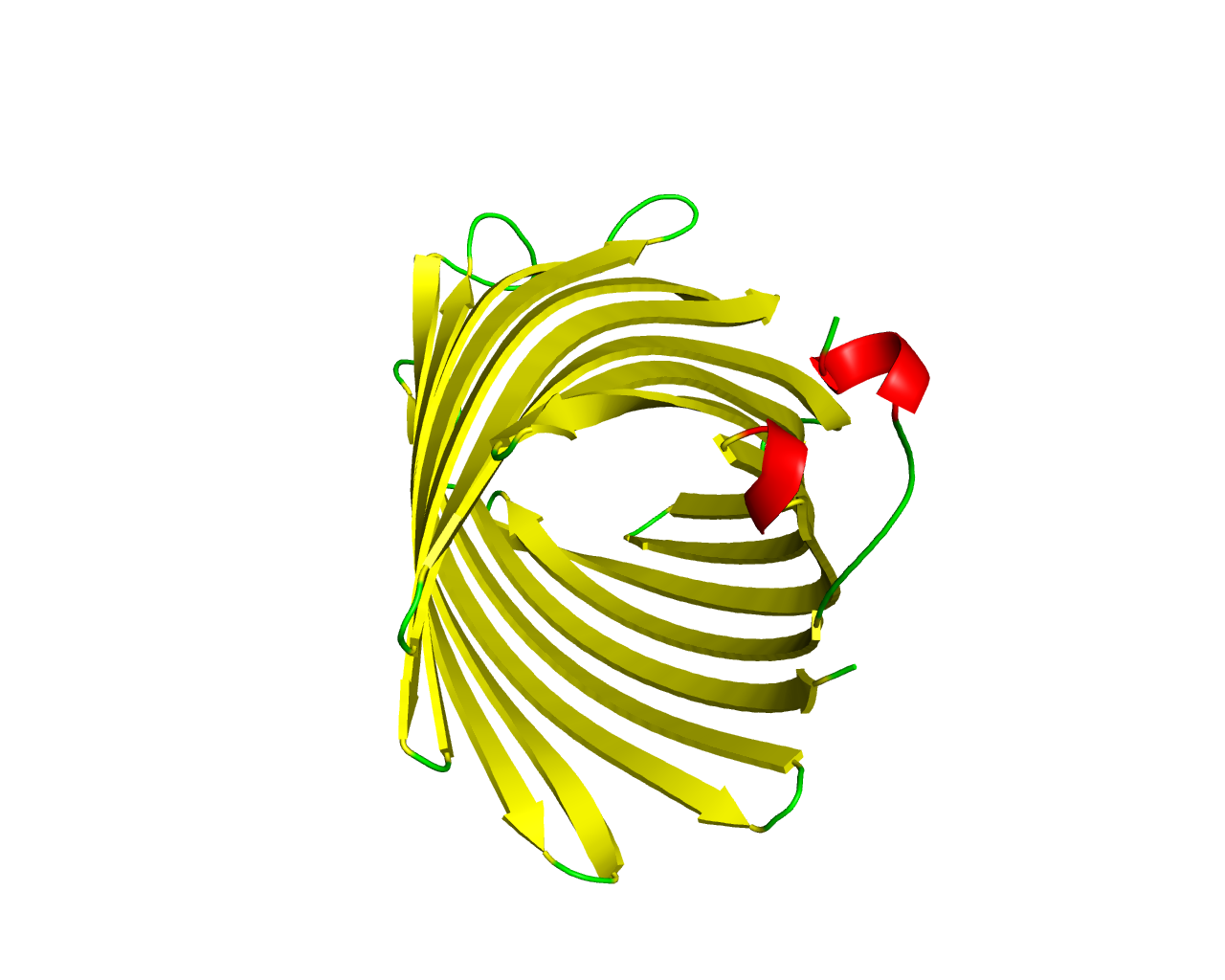 | ||||
| Literature references | ||||
Outer-membrane translocation of bulky small molecules by passive diffusion Proc Natl Acad Sci U S A. 2015 Jun 9;112(23):E2991-9. doi: 10.1073/pnas.1424835112. Epub 2015 May 26. PMID: 26015567 | ||||
CymA of Klebsiella oxytoca outer membrane: binding of cyclodextrins and study of the current noise of the open channel Biophys J. 2003 Aug;85(2):876-85. doi: 10.1016/S0006-3495(03)74527-5. PMID: 12885635 | ||||
Properties of a cyclodextrin-specific, unusual porin from Klebsiella oxytoca J Biol Chem. 1999 Aug 27;274(35):25159-66. doi: 10.1074/jbc.274.35.25159. PMID: 10455198 | ||||
| Proteins in this family with 3D-structure | ||||
View Entry | Description | Organism | Length | # strands |
