| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The Autotransporter beta-domain Family [Function: Biogenesis/Secretion] Seed alignment | Full alignment | Pfam Wiki page | TC-DB page | ||||
The type V secretion pathway encompasses the autotransporter proteins (AT-1), the two-partner secretion system (TPS) and the recently described type AT-2 family of proteins. AT proteins contain their own transporter domain, covalently attached to the C-terminal extremity of the secreted passenger domain. The secreted proteins are exported in a Sec-dependent manner across the inner membrane, after which they cross the outer membrane with the help of their cognate transporters. Translocation appears to be folding-sensitive, indicating that AT passenger domains cross the periplasm and the outer membrane in non-native conformations and fold progressively at the cell surface. A major difference between AT and TPS pathways arises from the manner by which specificity is established between the secreted protein and its transporter. To date, all of the functionally characterized autotransporters have been implicated in bacterial virulence by displaying enzymatic activity (protease, peptidase, lipase, esterase), mediating actin-promoted bacterial motility or acting as adhesins, toxins/cytotoxins, immunomodulatory proteins or, as more recently discovered, permitting maturation of another virulent protein. The structures of several translocator domains have been solved, suggesting that the &ß-barrel consists of 12 strands. FabF is a new class of secretion system similar to type V secretion in the autotransporter proteins (AT-1), characterized by a C-terminal, 12-stranded ß barrel with a helix-blocked pore in the closed state. However, in the truncated FapF structure, the N terminus exits the barrel on the periplasmic side rather than passing completely through the pore as in autotransporters. Regarding Hapb, it represents the ß barrel domain of self-associating autotransporters (SAATs). SAATs is a set of virulence factors that enhance bacterial biofilm 87. The Haemophilus influenzae Hap SAAT consists of three domains, the residues 1 - 25 correspond to the signal peptide, the residues 26 - 1036 refer to the Haps that called the passenger domain, while the residues 1037 - 1394 form the 14 ß strands at the C-terminal. | ||||
Representative image: 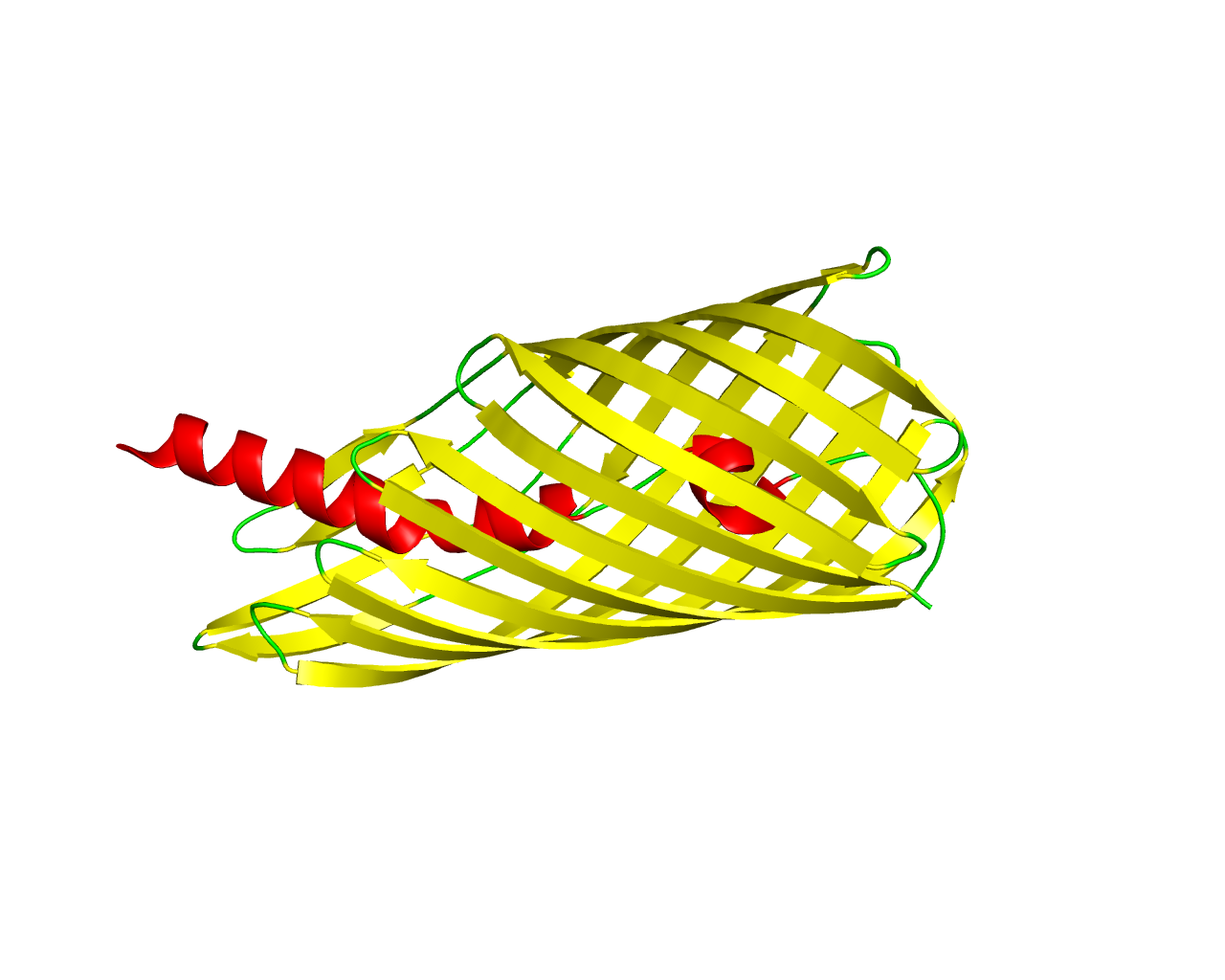 | ||||
| Literature references | ||||
BamA is required for autotransporter secretion Biochim Biophys Acta Gen Subj. 2020 Jul;1864(7):129581. doi: 10.1016/j.bbagen.2020.129581. Epub 2020 Feb 27. PMID: 32114025 | ||||
Comparison of type 5d autotransporter phospholipases demonstrates a correlation between high activity and intracellular pathogenic lifestyle Biochem J. 2019 Sep 24;476(18):2657-2676. doi: 10.1042/BCJ20190136. PMID: 31492736 | ||||
Biogenesis and function of the autotransporter adhesins YadA, intimin and invasin Int J Med Microbiol. 2019 Jul;309(5):331-337. doi: 10.1016/j.ijmm.2019.05.009. Epub 2019 Jun 1. PMID: 31176600 | ||||
Inhibition of autotransporter biogenesis by small molecules Mol Microbiol. 2019 Jul;112(1):81-98. doi: 10.1111/mmi.14255. Epub 2019 May 3. PMID: 30983025 | ||||
Type V Secretion in Gram-Negative Bacteria EcoSal Plus. 2019 Feb;8(2):10.1128/ecosalplus.ESP-0031-2018. doi: 10.1128/ecosalplus.ESP-0031-2018. PMID: 30838971 | ||||
Identification of a novel post-insertion step in the assembly of a bacterial outer membrane protein Mol Microbiol. 2018 Oct;110(1):143-159. doi: 10.1111/mmi.14102. Epub 2018 Sep 28. PMID: 30107065 | ||||
Hop-family Helicobacter outer membrane adhesins form a novel class of Type 5-like secretion proteins with an interrupted ß-barrel domain Mol Microbiol. 2018 Oct;110(1):33-46. doi: 10.1111/mmi.14075. Epub 2018 Sep 28. PMID: 29995350 | ||||
CapC, a Novel Autotransporter and Virulence Factor of Campylobacter jejuni Appl Environ Microbiol. 2018 Aug 1;84(16):e01032-18. doi: 10.1128/AEM.01032-18. Print 2018 Aug 15. PMID: 29915112 | ||||
Molecular basis for the folding of ß-helical autotransporter passenger domains Nat Commun. 2018 Apr 11;9(1):1395. doi: 10.1038/s41467-018-03593-2. PMID: 29643377 | ||||
Job contenders: roles of the ß-barrel assembly machinery and the translocation and assembly module in autotransporter secretion Mol Microbiol. 2017 Nov;106(4):505-517. doi: 10.1111/mmi.13832. Epub 2017 Sep 26. PMID: 28887826 | ||||
A new class of hybrid secretion system is employed in Pseudomonas amyloid biogenesis Nat Commun. 2017 Aug 15;8(1):263. doi: 10.1038/s41467-017-00361-6. PMID: 28811582 | ||||
Looks can be deceiving: recent insights into the mechanism of protein secretion by the autotransporter pathway Mol Microbiol. 2015 Jul;97(2):205-15. doi: 10.1111/mmi.13031. Epub 2015 May 15. PMID: 25881492 | ||||
The passenger-associated transport repeat promotes virulence factor secretion efficiency and delineates a distinct autotransporter subtype Mol Microbiol. 2015 Jul;97(2):315-29. doi: 10.1111/mmi.13027. Epub 2015 May 9. PMID: 25869731 | ||||
Analyzing the Role of Periplasmic Folding Factors in the Biogenesis of OMPs and Members of the Type V Secretion System Methods Mol Biol. 2015;1329:77-110. doi: 10.1007/978-1-4939-2871-2_7. PMID: 26427678 | ||||
Of linkers and autochaperones: an unambiguous nomenclature to identify common and uncommon themes for autotransporter secretion Mol Microbiol. 2015 Jan;95(1):1-16. doi: 10.1111/mmi.12838. Epub 2014 Nov 24. PMID: 25345653 | ||||
Crystal structure of the Haemophilus influenzae Hap adhesin reveals an intercellular oligomerization mechanism for bacterial aggregation EMBO J. 2011 Aug 12;30(18):3864-74. doi: 10.1038/emboj.2011.279. PMID: 21841773 | ||||
Autotransporter passenger domain secretion requires a hydrophobic cavity at the extracellular entrance of the ß-domain pore Biochem J. 2011 May 1;435(3):577-87. doi: 10.1042/BJ20101548. PMID: 21306302 | ||||
Crystal structure of a full-length autotransporter J Mol Biol. 2010 Feb 26;396(3):627-33. doi: 10.1016/j.jmb.2009.12.061. Epub 2010 Jan 11. PMID: 20060837 | ||||
Autotransporter structure reveals intra-barrel cleavage followed by conformational changes Nat Struct Mol Biol. 2007 Dec;14(12):1214-20. doi: 10.1038/nsmb1322. Epub 2007 Nov 11. PMID: 17994105 | ||||
Type V protein secretion pathway: the autotransporter story Microbiol Mol Biol Rev. 2004 Dec;68(4):692-744. doi: 10.1128/MMBR.68.4.692-744.2004. PMID: 15590781 | ||||
Structure of the translocator domain of a bacterial autotransporter EMBO J. 2004 Mar 24;23(6):1257-66. doi: 10.1038/sj.emboj.7600148. Epub 2004 Mar 11. PMID: 15014442 | ||||
The autotransporter secretion system Res Microbiol. 2004 Mar;155(2):53-60. doi: 10.1016/j.resmic.2003.10.002. PMID: 14990256 | ||||
Autotransporter and two-partner secretion: delivery of large-size virulence factors by gram-negative bacterial pathogens Crit Rev Microbiol. 2004;30(4):275-86. doi: 10.1080/10408410490499872. PMID: 15646401 | ||||
Structural determinants of processing and secretion of the Haemophilus influenzae hap protein Mol Microbiol. 1997 Nov;26(3):505-18. doi: 10.1046/j.1365-2958.1997.5921965.x. PMID: 9402021 | ||||
| Proteins in this family with 3D-structure | ||||
View Entry | Description | Organism | Length | # strands |
