| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The YadA-like membrane anchor domain (Autotransporter-2) Family [Function: Biogenesis/Secretion] Seed alignment | Full alignment | Pfam page | TC-DB page | ||||
The type V secretion pathway encompasses the autotransporter proteins (AT-1), the two-partner secretion system (TPS) and the recently described type AT-2 family of proteins. The prototype of the last family is the adhesin A (YadA) of Yersinia enterocolitica. Homologs are found in a variety of pathogenic bacteria. Proteins of this family mediate attachment to the surfaces of host cells and protect bacteria against complement and the bactericidal activities of defensins. Adhesins of this family form oligomeric lollipop-like structures anchored in the outer membrane by their C-termini. In contrast to the typical autotransporters, whose C-terminal porin regions are of about 250 residues long and possess 12 transmembrane ß strands, members of the AT-2 family, have their C-terminal pore-forming anchors composed of about only 70 residues, with just 4 possible transmembrane ß strands and form trimers. These facts suggest that the pore-forming translocator units evolved independently and they belong to distinct families. The structure of one such protein, the HiA autotransporter of Haemophilus influenzae has been solved revealing a ß-barrel with 12 transmembrane ß-strands, comprised by 4 strands from each subunit. The central channel has a pore of 1.8 nm in diameter that is traversed by three N-terminal alpha helices, one from each subunit. | ||||
Representative image: 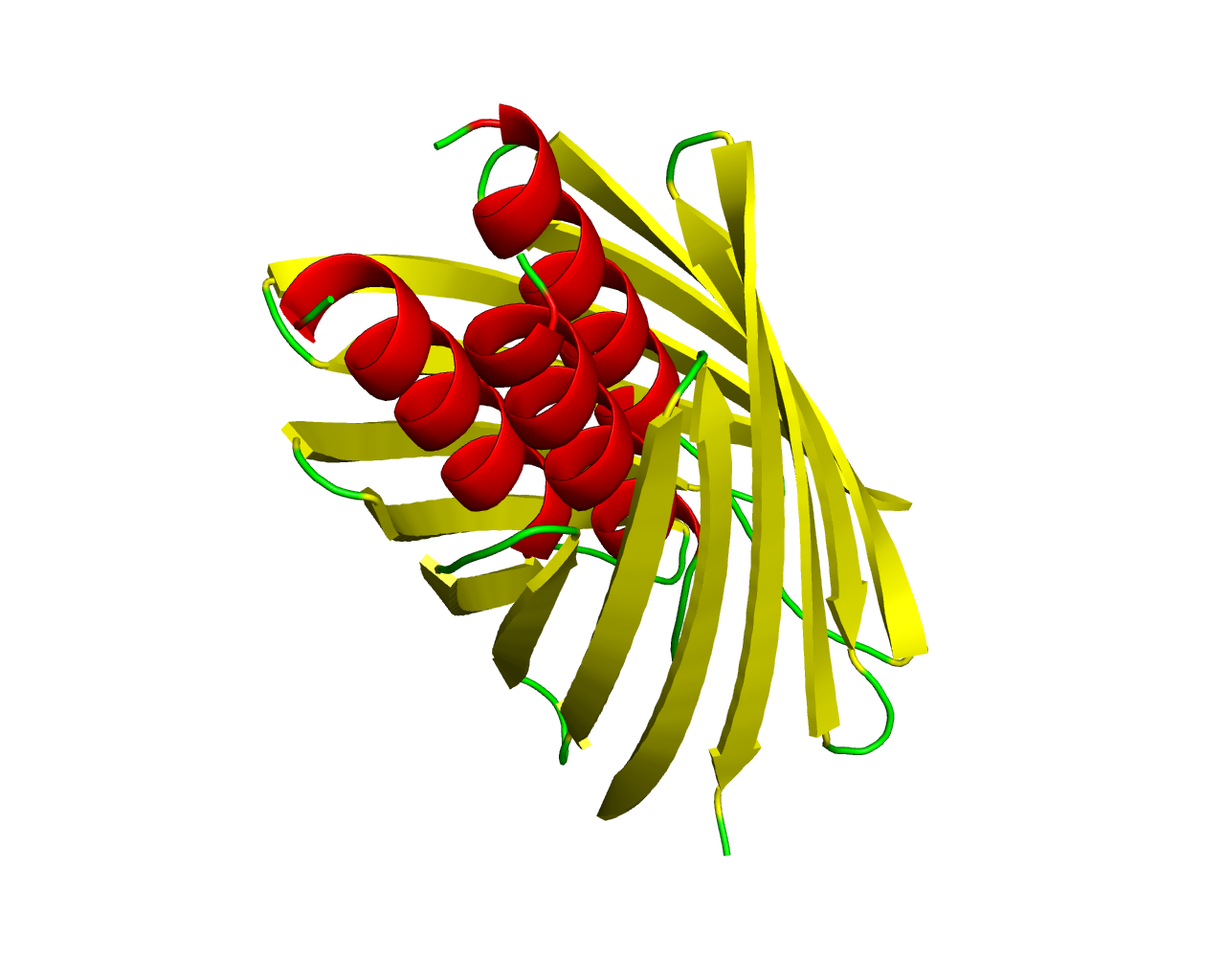 | ||||
| Literature references | ||||
Biogenesis and function of the autotransporter adhesins YadA, intimin and invasin Int J Med Microbiol. 2019 Jul;309(5):331-337. doi: 10.1016/j.ijmm.2019.05.009. Epub 2019 Jun 1. PMID: 31176600 | ||||
In vitro assembly of Haemophilus influenzae adhesin transmembrane domain and studies on the electrostatic repulsion at the interface Biophys Rev. 2019 Jun;11(3):303-309. doi: 10.1007/s12551-019-00535-0. Epub 2019 May 9. PMID: 31073957 | ||||
Electrostatic Repulsion between Unique Arginine Residues Is Essential for the Efficient in Vitro Assembly of the Transmembrane Domain of a Trimeric Autotransporter Biochemistry. 2017 Apr 18;56(15):2139-2148. doi: 10.1021/acs.biochem.6b01130. Epub 2017 Apr 10. PMID: 28357859 | ||||
Yeast Mitochondria as a Model System to Study the Biogenesis of Bacterial ß-Barrel Proteins Methods Mol Biol. 2015;1329:17-31. doi: 10.1007/978-1-4939-2871-2_2. PMID: 26427673 | ||||
Yersinia adhesin A (YadA)--beauty & beast Int J Med Microbiol. 2015 Feb;305(2):252-8. doi: 10.1016/j.ijmm.2014.12.008. Epub 2014 Dec 24. PMID: 25604505 | ||||
Evolutionary conservation in biogenesis of ß-barrel proteins allows mitochondria to assemble a functional bacterial trimeric autotransporter protein J Biol Chem. 2014 Oct 24;289(43):29457-70. doi: 10.1074/jbc.M114.565655. Epub 2014 Sep 4. PMID: 25190806 | ||||
Yersinia infection tools-characterization of structure and function of adhesins Front Cell Infect Microbiol. 2013 Jan 8;2:169. doi: 10.3389/fcimb.2012.00169. eCollection 2012. PMID: 23316485 | ||||
Stability and membrane interactions of an autotransport protein: MD simulations of the Hia translocator domain in a complex membrane environment Biochim Biophys Acta. 2013 Feb;1828(2):715-23. doi: 10.1016/j.bbamem.2012.09.002. Epub 2012 Sep 13. PMID: 22982599 | ||||
The translocation domain in trimeric autotransporter adhesins is necessary and sufficient for trimerization and autotransportation J Bacteriol. 2012 Feb;194(4):827-38. doi: 10.1128/JB.05322-11. Epub 2011 Dec 9. PMID: 22155776 | ||||
Mitochondria can recognize and assemble fragments of a beta-barrel structure Mol Biol Cell. 2011 May 15;22(10):1638-47. doi: 10.1091/mbc.E10-12-0943. Epub 2011 Apr 1. PMID: 21460184 | ||||
Structure and biology of trimeric autotransporter adhesins Adv Exp Med Biol. 2011;715:143-58. doi: 10.1007/978-94-007-0940-9_9. PMID: 21557062 | ||||
Structure of the outer membrane translocator domain of the Haemophilus influenzae Hia trimeric autotransporter EMBO J. 2006 Jun 7;25(11):2297-304. doi: 10.1038/sj.emboj.7601132. Epub 2006 May 11. PMID: 16688217 | ||||
The Haemophilus influenzae Hia autotransporter contains an unusually short trimeric translocator domain J Biol Chem. 2004 Apr 9;279(15):14679-85. doi: 10.1074/jbc.M311496200. Epub 2004 Jan 15. PMID: 14726537 | ||||
Molecular analysis of transport and oligomerization of the Yersinia enterocolitica adhesin YadA J Bacteriol. 2003 Jul;185(13):3735-44. doi: 10.1128/jb.185.13.3735-3744.2003. PMID: 12813066 | ||||
YadA, the multifaceted Yersinia adhesin Int J Med Microbiol. 2001 Aug;291(3):209-18. doi: 10.1078/1438-4221-00119. PMID: 11554561 | ||||

|

|

|

|
