| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The Phospholipase A1 (PLA1) Family [Function: Enzyme] Seed alignment | Full alignment | Pfam page | Pfam Wiki page | TC-DB page | ||||
Members of the Outer membrane phospholipase A (OMPLA) family are integral membrane phospholipases, present in many Gram-negative bacteria with a broad substrate specificity. The role of OMPLA has been most thoroughly studied in Escherichia coli, where it participates in the secretion of colicins. The three-dimensional structure of OmplA of Escherichia coli has been determined, revealing a 12-stranded antiparallel ß-barrel with a convex and a flat side. The active site residues are exposed at the exterior of the flat face of the barrel. The activity of the enzyme is calcium-dependent and regulated by reversible dimerisation. Dimer interactions occur exclusively in the membrane-embedded parts of the flat side of the barrel, with polar residues embedded in an apolar environment forming the key interactions. The active site (Asn-156-His-142-Ser-144) is located at the exterior of the barrel, at the outer leaflet side of the membrane. The phospholipids of the inner leaflet are believed to be presented to the active site of OMPLA by perturbation of the membrane causing the formation of non-bilayer structures and finally leading to the formation of the active dimer. | ||||
Representative image: 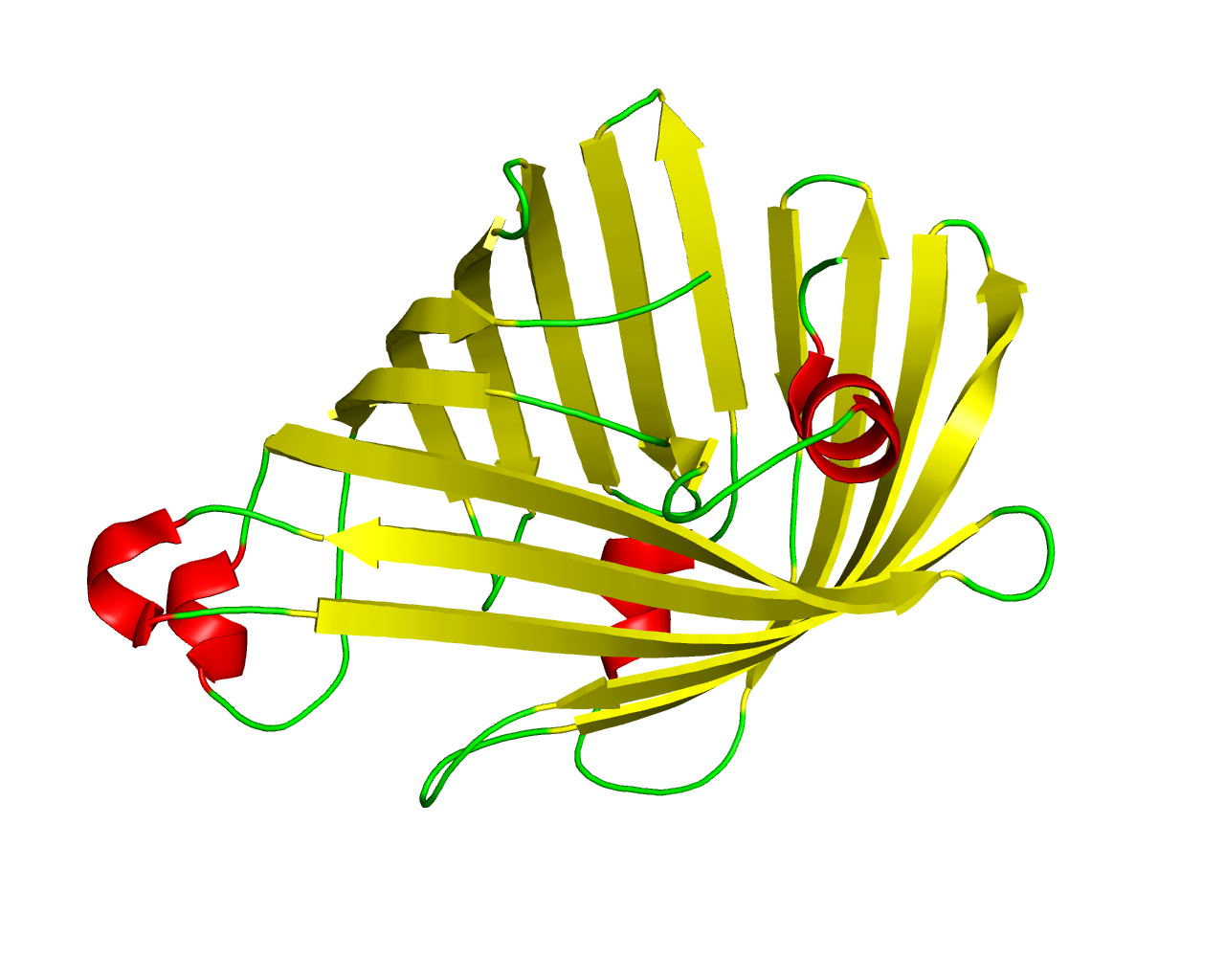 | ||||
| Literature references | ||||
Influence of Protein Scaffold on Side-Chain Transfer Free Energies Biophys J. 2017 Aug 8;113(3):597-604. doi: 10.1016/j.bpj.2017.06.032. PMID: 28793214 | ||||
Outer membrane phospholipase A's roles in Helicobacter pylori acid adaptation Gut Pathog. 2017 Jun 12;9:36. doi: 10.1186/s13099-017-0184-y. eCollection 2017. PMID: 28616083 | ||||
Outer Membrane Protein Folding and Topology from a Computational Transfer Free Energy Scale J Am Chem Soc. 2016 Mar 2;138(8):2592-601. doi: 10.1021/jacs.5b10307. Epub 2016 Feb 19. PMID: 26860422 | ||||
Interaction of phospholipase A of the E. coli outer membrane with the inhibitors of eucaryotic phospholipases A₂ and their effect on the Ca²⁺-induced permeabilization of the bacterial membrane J Membr Biol. 2014 Mar;247(3):281-8. doi: 10.1007/s00232-014-9633-4. Epub 2014 Jan 30. PMID: 24477786 | ||||
Structural biology of membrane-intrinsic beta-barrel enzymes: sentinels of the bacterial outer membrane Biochim Biophys Acta. 2008 Sep;1778(9):1881-96. doi: 10.1016/j.bbamem.2007.07.021. Epub 2007 Aug 11. PMID: 17880914 | ||||
Detergent organisation in crystals of monomeric outer membrane phospholipase A J Struct Biol. 2003 Feb;141(2):122-31. doi: 10.1016/s1047-8477(02)00579-8. PMID: 12615538 | ||||
Structural investigations of the active-site mutant Asn156Ala of outer membrane phospholipase A: function of the Asn-His interaction in the catalytic triad Protein Sci. 2001 Oct;10(10):1962-9. doi: 10.1110/ps.17701. PMID: 11567087 | ||||
Structural investigations of calcium binding and its role in activity and activation of outer membrane phospholipase A from Escherichia coli J Mol Biol. 2001 Jun 1;309(2):477-89. doi: 10.1006/jmbi.2001.4675. PMID: 11371166 | ||||
Bacterial phospholipase A: structure and function of an integral membrane phospholipase Biochim Biophys Acta. 2000 Oct 31;1488(1-2):91-101. doi: 10.1016/s1388-1981(00)00113-x. PMID: 11080680 | ||||
Structural evidence for dimerization-regulated activation of an integral membrane phospholipase Nature. 1999 Oct 14;401(6754):717-21. doi: 10.1038/44890. PMID: 10537112 | ||||
Bacteriocin release protein triggers dimerization of outer membrane phospholipase A in vivo J Bacteriol. 1999 May;181(10):3281-3. doi: 10.1128/JB.181.10.3281-3283.1999. PMID: 10322034 | ||||
| Proteins in this family with 3D-structure | ||||
View Entry | Description | Organism | Length | # strands |
