| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The Antimicrobial peptide resistance and lipid A acylation protein (PagP) Family [Function: Enzyme] Seed alignment | Full alignment | Pfam page | Pfam Wiki page | ||||
This family consists of several bacterial antimicrobial peptide resistance and lipid A acylation (PagP) proteins. In a number of pathogenic Gram-negative bacteria, PagP offers resistance to certain cationic antimicrobial peptides produced during the host innate immune response. PagP is an integral outer membrane enzyme (acyltransferase) that transfers a palmitate chain from a phospholipid to lipid A (endotoxin). PagP is indispensable for lipid A palmitoylation in Gram-negative bacteria and has been implicated in resistance to host immune defenses. PagP possesses an unusual structure for an integral membrane protein, with a highly dynamic barrel domain that is tilted with respect to the membrane normal. PagP can distinguish lipid acyl chains that differ by a single methylene unit, indicating that the enzyme possesses a remarkably precise hydrocarbon ruler. The crystal structure of PagP from Escherichia coli has been solved, suggesting an 8-stranded ß-barrel, preceded by an amphipathic alpha helix, with an interior hydrophobic pocket occupied by a single detergent molecule. Acyl-group specificity is modulated by mutation of Gly(88) lining the bottom of the hydrophobic pocket and therefore confirming the hydrocarbon ruler mechanism for palmitate recognition. Three amino acid residues, critical for enzymatic activity are localized to extracellular loops in order to interact with the polar head groups of lipid A. Hence, the active site of PagP is situated on the outer surface of the outer membrane, similar to the outer membrane phospholipases. | ||||
Representative image: 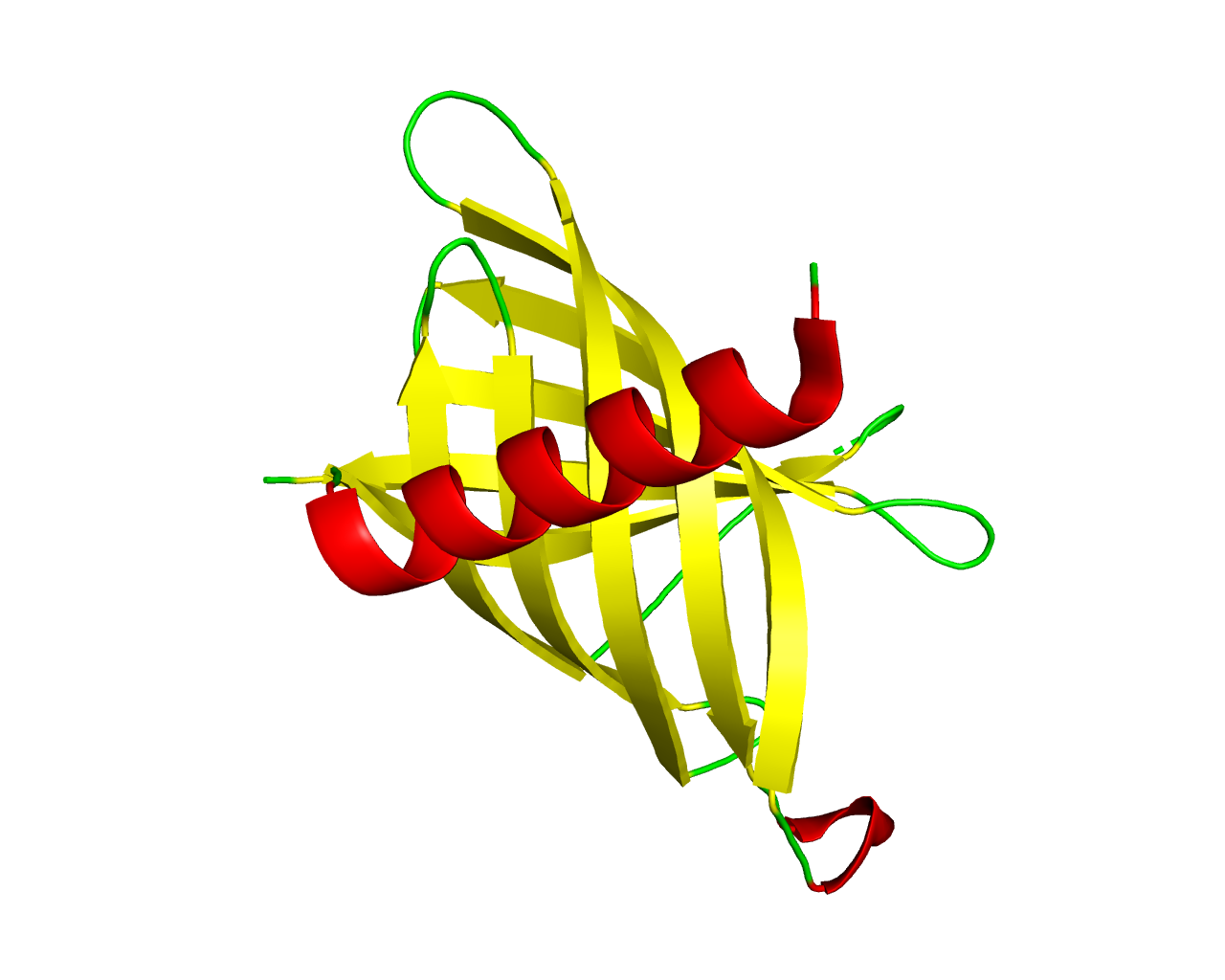 | ||||
| Literature references | ||||
Hydrophobic Characteristic Is Energetically Preferred for Cysteine in a Model Membrane Protein Biophys J. 2019 Jul 9;117(1):25-35. doi: 10.1016/j.bpj.2019.05.024. Epub 2019 Jun 5. PMID: 31221440 | ||||
Salvaging the Thermodynamic Destabilization of Interface Histidine in Transmembrane ß-Barrels Biochemistry. 2018 Dec 4;57(48):6669-6678. doi: 10.1021/acs.biochem.8b00805. Epub 2018 Oct 16. PMID: 30284812 | ||||
Influence of Protein Scaffold on Side-Chain Transfer Free Energies Biophys J. 2017 Aug 8;113(3):597-604. doi: 10.1016/j.bpj.2017.06.032. PMID: 28793214 | ||||
Energetics of side-chain partitioning of ß-signal residues in unassisted folding of a transmembrane ß-barrel protein J Biol Chem. 2017 Jul 21;292(29):12351-12365. doi: 10.1074/jbc.M117.789446. Epub 2017 Jun 7. PMID: 28592485 | ||||
Distinct Structural Elements Govern the Folding, Stability, and Catalysis in the Outer Membrane Enzyme PagP Biochemistry. 2016 Sep 6;55(35):4960-70. doi: 10.1021/acs.biochem.6b00678. Epub 2016 Aug 24. PMID: 27525547 | ||||
Residue-Dependent Thermodynamic Cost and Barrel Plasticity Balances Activity in the PhoPQ-Activated Enzyme PagP of Salmonella typhimurium Biochemistry. 2015 Sep 22;54(37):5712-22. doi: 10.1021/acs.biochem.5b00543. Epub 2015 Sep 10. PMID: 26334694 | ||||
A divergent Pseudomonas aeruginosa palmitoyltransferase essential for cystic fibrosis-specific lipid A Mol Microbiol. 2014 Jan;91(1):158-74. doi: 10.1111/mmi.12451. Epub 2013 Nov 27. PMID: 24283944 | ||||
Dissecting the effects of periplasmic chaperones on the in vitro folding of the outer membrane protein PagP J Mol Biol. 2013 Sep 9;425(17):3178-91. doi: 10.1016/j.jmb.2013.06.017. Epub 2013 Jun 22. PMID: 23796519 | ||||
A PagP fusion protein system for the expression of intrinsically disordered proteins in Escherichia coli Protein Expr Purif. 2012 Sep;85(1):148-51. doi: 10.1016/j.pep.2012.07.007. Epub 2012 Jul 25. PMID: 22841980 | ||||
One membrane protein, two structures and six environments: a comparative molecular dynamics simulation study of the bacterial outer membrane protein PagP Mol Membr Biol. 2009 May;26(4):205-14. doi: 10.1080/09687680902788967. Epub 2009 Mar 10. PMID: 19280380 | ||||
Structural biology of membrane-intrinsic beta-barrel enzymes: sentinels of the bacterial outer membrane Biochim Biophys Acta. 2008 Sep;1778(9):1881-96. doi: 10.1016/j.bbamem.2007.07.021. Epub 2007 Aug 11. PMID: 17880914 | ||||
A hydrocarbon ruler measures palmitate in the enzymatic acylation of endotoxin EMBO J. 2004 Aug 4;23(15):2931-41. doi: 10.1038/sj.emboj.7600320. Epub 2004 Jul 22. PMID: 15272304 | ||||
Solution structure and dynamics of the outer membrane enzyme PagP by NMR Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13560-5. doi: 10.1073/pnas.212344499. Epub 2002 Sep 30. PMID: 12357033 | ||||
| Proteins in this family with 3D-structure | ||||
View Entry | Description | Organism | Length | # strands |
