| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The Outer membrane protein OpcA Family [Function: Adhesion] Seed alignment | Full alignment | Pfam page | Pfam Wiki page | TC-DB page | ||||
This family consists of several adhesins found in Neisseria species. OpcA is an integral outer membrane protein from Neisseria meningitidis, the causative agent of meningococcal meningitis and septicemia. It mediates the adhesion of Neisseria meningitidis to epithelial and endothelial cells by binding to vitronectin and proteoglycan cell-surface receptors. The crystal structure of OpcA has been determined to 2.0 A resolution, showing a 10-stranded ß-barrel structure with extensive loop regions that protrude above the predicted surface of the membrane. Loops 2, 3, 4 and 5 associate to form one side of a crevice in the external surface of the structure, the other side being formed by loop 1. OpcA is one of the Major Outer Membrane Proteins and has been shown to play an important role in meningococcal adhesion and invasion of both epithelial and endothelial cells. | ||||
Representative image: 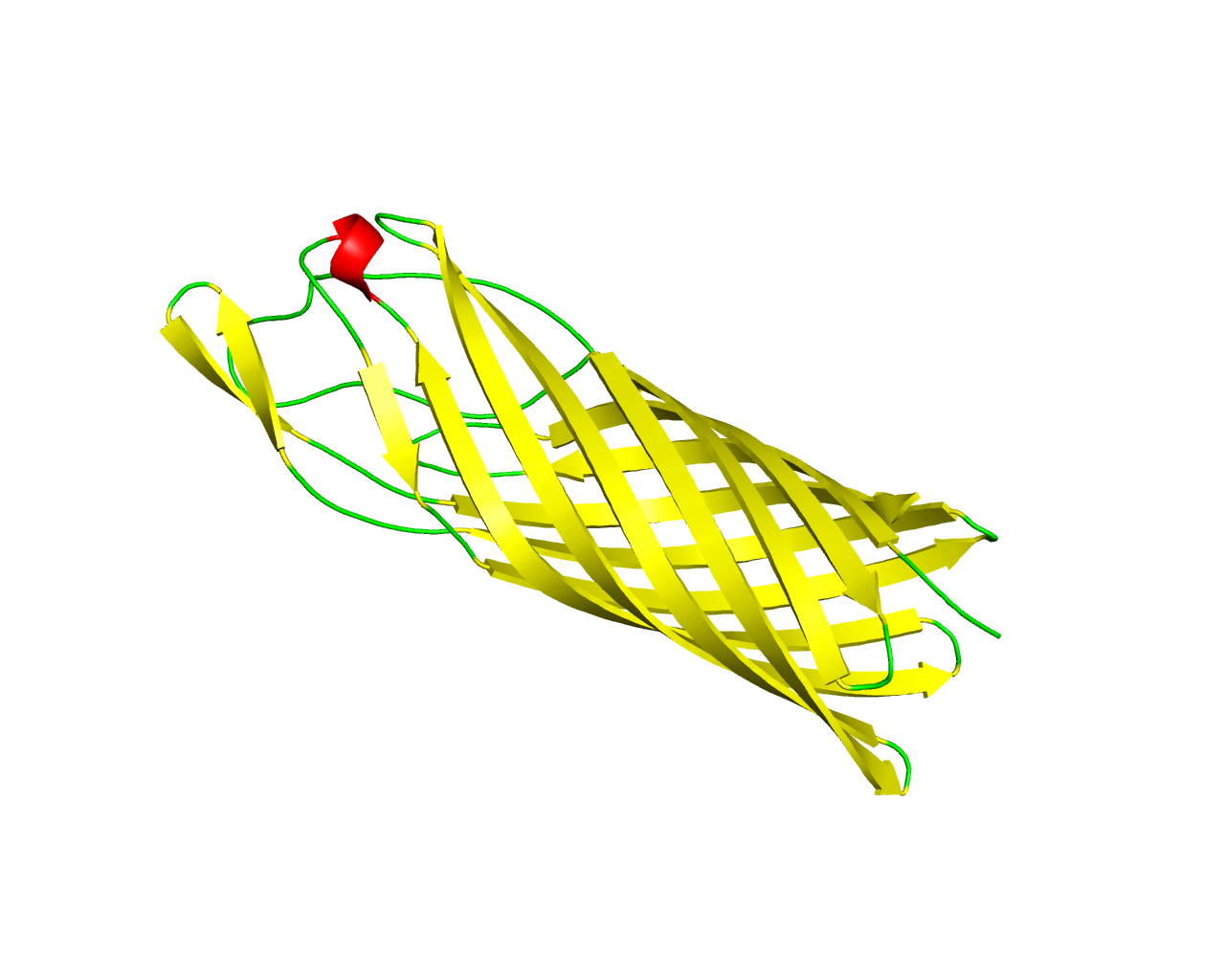 | ||||
| Literature references | ||||
Outer membrane vesicles extracted from Neisseria meningitidis serogroup X for prevention of meningococcal disease in Africa Pharmacol Res. 2017 Jul;121:194-201. doi: 10.1016/j.phrs.2017.04.030. Epub 2017 May 8. PMID: 28495657 | ||||
Functional fragments of disorder in outer membrane ß barrel proteins Intrinsically Disord Proteins. 2013 Apr 1;1(1):e24848. doi: 10.4161/idp.24848. eCollection 2013 Jan-Dec. PMID: 28516011 | ||||
Analysis of the bactericidal response to an experimental Neisseria meningitidis vesicle vaccine Clin Vaccine Immunol. 2012 May;19(5):659-65. doi: 10.1128/CVI.00070-12. Epub 2012 Mar 29. PMID: 22461527 | ||||
A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over-expressed factor H binding protein, two PorAs and stabilized OpcA expression Vaccine. 2011 Feb 4;29(7):1413-20. doi: 10.1016/j.vaccine.2010.12.039. Epub 2011 Jan 1. PMID: 21199704 | ||||
Identification of opcA gene in Neisseria polysaccharea: interspecies diversity of Opc protein family Gene. 2003 Mar 27;307:31-40. doi: 10.1016/s0378-1119(02)01208-8. PMID: 12706886 | ||||
Crystal structure of the OpcA integral membrane adhesin from Neisseria meningitidis Proc Natl Acad Sci U S A. 2002 Mar 19;99(6):3417-21. doi: 10.1073/pnas.062630899. Epub 2002 Mar 12. PMID: 11891340 | ||||
| Proteins in this family with 3D-structure | ||||
View Entry | Description | Organism | Length | # strands |
