| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The Oligogalacturonate-specific porin protein (KdgM) Family [Function: Specific diffusion channels] Seed alignment | Full alignment | Pfam page | Pfam Wiki page | TC-DB page | ||||
This family consists of several bacterial proteins which are homologous to the oligogalacturonate-specific porin protein KdgM from Erwinia chrysanthemi. This phytopathogenic Gram-negative bacterium secretes pectinases, which are able to degrade the pectic polymers of plant cell walls and uses the degradation products as a carbon source for growth. KdgM is a Major Outer Membrane Protein (MOMP), whose synthesis is induced in the presence of the pectic derivatives. KdgM behaves like a voltage-dependent porin that is slightly selective for anions and that exhibits fast block in the presence of trigalacturonate. The family members seem to be monomeric, while the determined 3D structure of NanC porin suggests that they possess a 12-stranded ß-barrel, with rather short extracellular loops and a larger one that restricts the size of the pore. An outer membrane porin, KdgM, is indispensable for the uptake of acidic oligosaccharides. KdgM is folded into a regular 12-stranded antiparallel ß-barrel with a circular cross-section defining a transmembrane pore with a minimal radius of 3.1 Å. | ||||
Representative image: 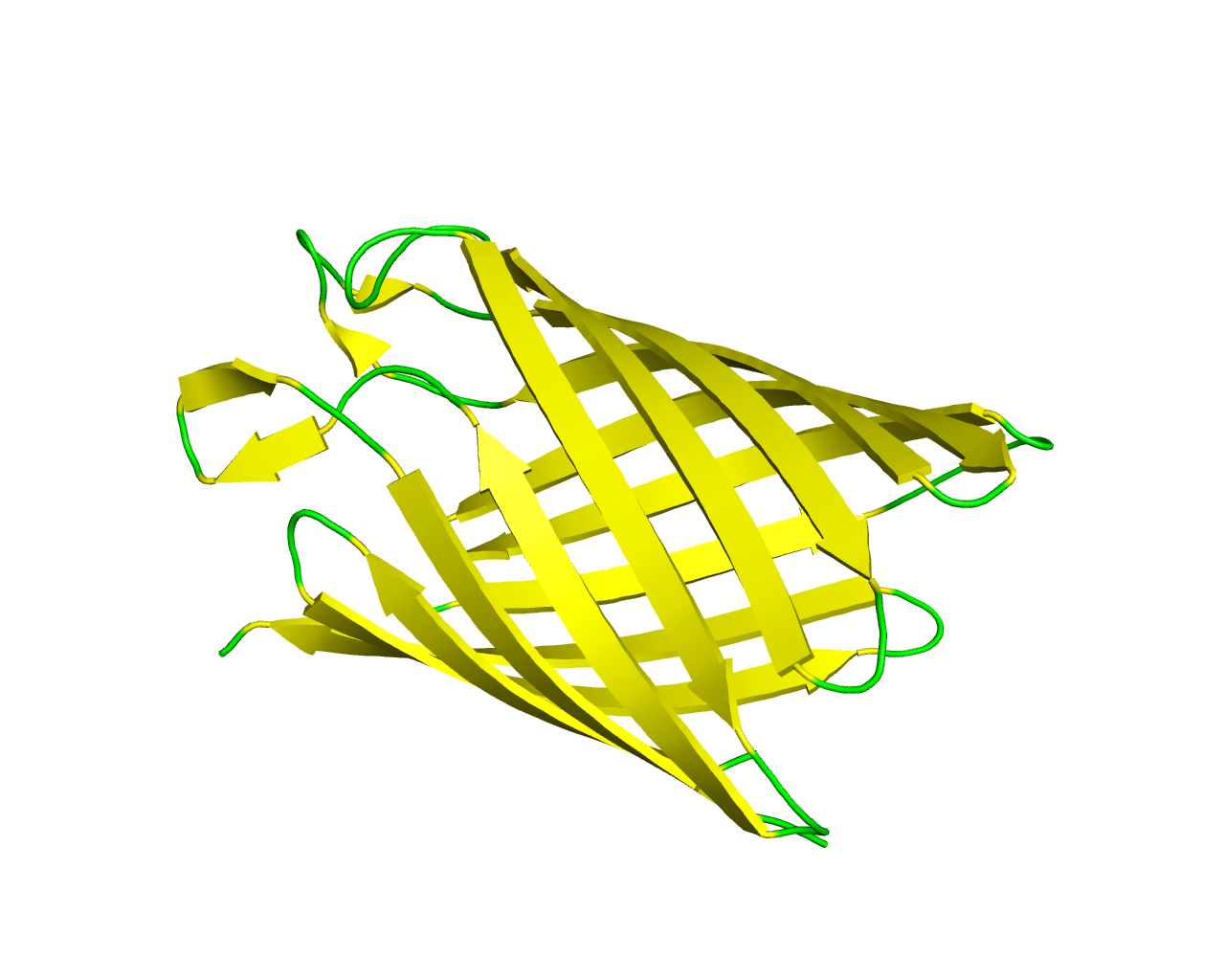 | ||||
| Literature references | ||||
Structure of the oligogalacturonate-specific KdgM porin Acta Crystallogr D Biol Crystallogr. 2014 Jun;70(Pt 6):1770-8. doi: 10.1107/S1399004714007147. Epub 2014 May 30. PMID: 24914987 | ||||
The free energy landscape of dimerization of a membrane protein, NanC PLoS Comput Biol. 2014 Jan;10(1):e1003417. doi: 10.1371/journal.pcbi.1003417. Epub 2014 Jan 9. PMID: 24415929 | ||||
Simulation of ion transport through an N-acetylneuraminic acid-inducible membrane channel: from understanding to engineering J Phys Chem B. 2013 Dec 19;117(50):15966-75. doi: 10.1021/jp408495v. Epub 2013 Nov 14. PMID: 24161028 | ||||
Ion permeation in the NanC porin from Escherichia coli: free energy calculations along pathways identified by coarse-grain simulations J Phys Chem B. 2013 Oct 31;117(43):13534-42. doi: 10.1021/jp4081838. Epub 2013 Oct 22. PMID: 24147565 | ||||
Single-channel measurements of an N-acetylneuraminic acid-inducible outer membrane channel in Escherichia coli Eur Biophys J. 2012 Mar;41(3):259-71. doi: 10.1007/s00249-011-0781-5. Epub 2012 Jan 13. PMID: 22246445 | ||||
Loss of elongation factor P disrupts bacterial outer membrane integrity J Bacteriol. 2012 Jan;194(2):413-25. doi: 10.1128/JB.05864-11. Epub 2011 Nov 11. PMID: 22081389 | ||||
OmpK26, a novel porin associated with carbapenem resistance in Klebsiella pneumoniae Antimicrob Agents Chemother. 2011 Oct;55(10):4742-7. doi: 10.1128/AAC.00309-11. Epub 2011 Aug 1. PMID: 21807980 | ||||
NanC crystal structure, a model for outer-membrane channels of the acidic sugar-specific KdgM porin family J Mol Biol. 2009 Dec 11;394(4):718-31. doi: 10.1016/j.jmb.2009.09.054. Epub 2009 Sep 29. PMID: 19796645 | ||||
Projection maps of three members of the KdgM outer membrane protein family J Struct Biol. 2007 Dec;160(3):395-403. doi: 10.1016/j.jsb.2007.08.007. Epub 2007 Aug 23. PMID: 17919922 | ||||
Function and expression of an N-acetylneuraminic acid-inducible outer membrane channel in Escherichia coli J Bacteriol. 2005 Mar;187(6):1959-65. doi: 10.1128/JB.187.6.1959-1965.2005. PMID: 15743943 | ||||
Topology of the Erwinia chrysanthemi oligogalacturonate porin KdgM Biochem J. 2003 Jun 1;372(Pt 2):329-34. doi: 10.1042/BJ20030027. PMID: 12603200 | ||||
The oligogalacturonate-specific porin KdgM of Erwinia chrysanthemi belongs to a new porin family J Biol Chem. 2002 Mar 8;277(10):7936-44. doi: 10.1074/jbc.M109193200. Epub 2001 Dec 28. PMID: 11773048 | ||||
| Proteins in this family with 3D-structure | ||||
View Entry | Description | Organism | Length | # strands |
