| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The Phosphate-selective porin O and P Family [Function: Specific diffusion channels] Seed alignment | Full alignment | Pfam page | Pfam Wiki page | TC-DB page | ||||
This family is comprised by proteins similar to the phosphate-selective porins O and P, which are found mainly in Pseudomonas aeruginosa. These anion-specific porins possess a binding site that has a higher affinity to phosphate than chloride ions. Porin O (OprO) has a higher affinity to polyphosphates, while porin P (OprP) has a higher affinity to orthophosphate. In Pseudomonas aeruginosa, porin O was found to be expressed only under phosphate-starvation conditions during the stationary growth phase. The crystal structure of OprP has revealed a 16-stranded ß-barrel similar to other general diffusion porins. | ||||
Representative image: 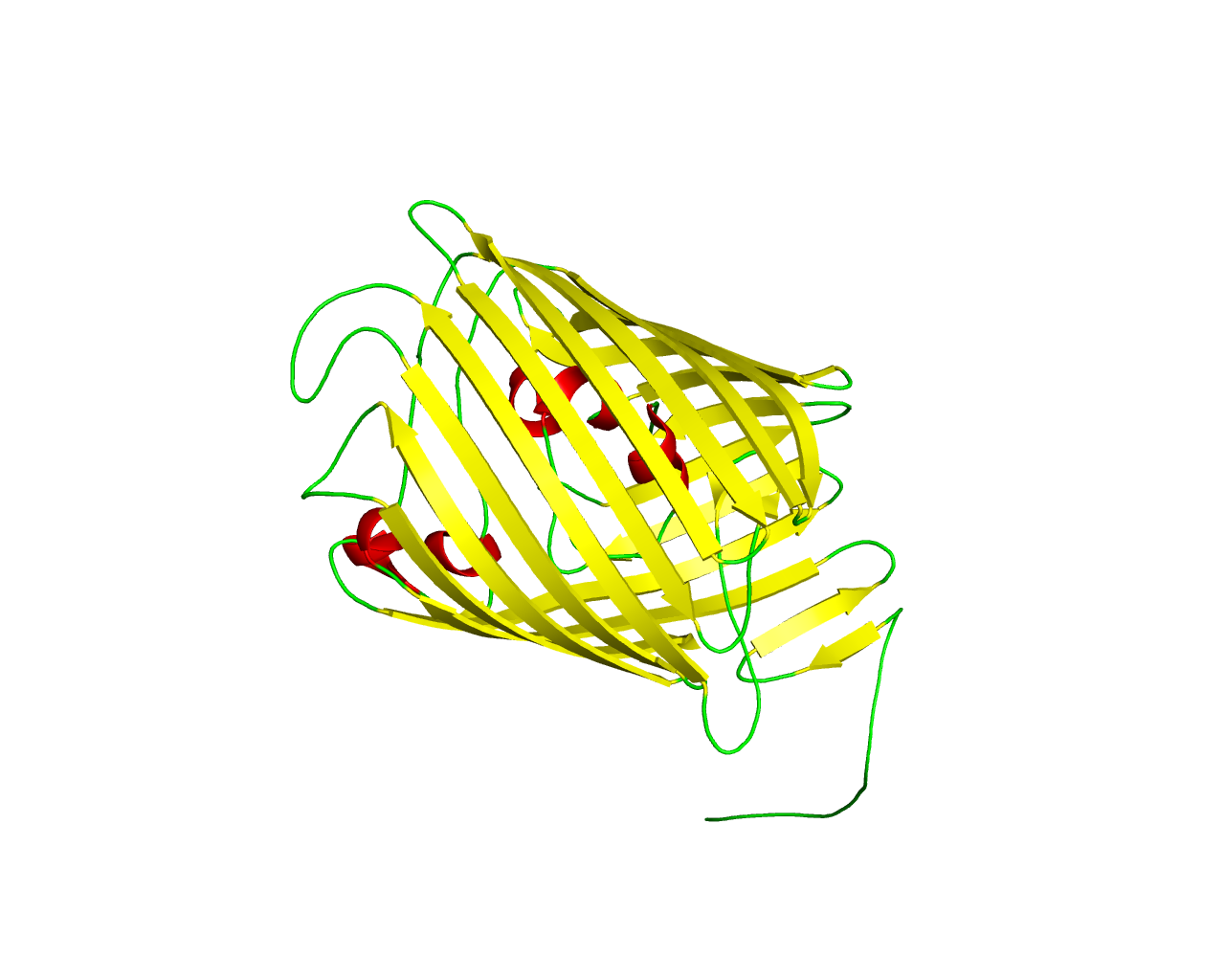 | ||||
| Literature references | ||||
Probing transport of fosfomycin through substrate specific OprO and OprP from Pseudomonas aeruginosa Biochem Biophys Res Commun. 2018 Jan 1;495(1):1454-1460. doi: 10.1016/j.bbrc.2017.11.188. Epub 2017 Dec 6. PMID: 29198700 | ||||
Structure, function and regulation of Pseudomonas aeruginosa porins FEMS Microbiol Rev. 2017 Sep 1;41(5):698-722. doi: 10.1093/femsre/fux020. PMID: 28981745 | ||||
Conversion of OprO into an OprP-like Channel by Exchanging Key Residues in the Channel Constriction Biophys J. 2017 Aug 22;113(4):829-834. doi: 10.1016/j.bpj.2017.07.004. PMID: 28834719 | ||||
Why do the outer membrane proteins OmpF from E. coli and OprP from P. aeruginosa prefer trimers? Simulation studies J Mol Graph Model. 2016 Apr;65:1-7. doi: 10.1016/j.jmgm.2016.02.002. Epub 2016 Feb 10. PMID: 26895142 | ||||
Structure, Dynamics, and Substrate Specificity of the OprO Porin from Pseudomonas aeruginosa Biophys J. 2015 Oct 6;109(7):1429-38. doi: 10.1016/j.bpj.2015.07.035. PMID: 26445443 | ||||
Role of the central arginine R133 toward the ion selectivity of the phosphate specific channel OprP: effects of charge and solvation Biochemistry. 2013 Aug 20;52(33):5522-32. doi: 10.1021/bi400522b. Epub 2013 Aug 9. PMID: 23875754 | ||||
An arginine ladder in OprP mediates phosphate-specific transfer across the outer membrane Nat Struct Mol Biol. 2007 Jan;14(1):85-7. doi: 10.1038/nsmb1189. Epub 2006 Dec 24. PMID: 17187075 | ||||
Anion transport through the phosphate-specific OprP-channel of the Pseudomonas aeruginosa outer membrane: effects of phosphate, di- and tribasic anions and of negatively-charged lipids Biochim Biophys Acta. 1993 Jul 4;1149(2):224-30. doi: 10.1016/0005-2736(93)90205-e. PMID: 8323941 | ||||
Polyphosphate-selective porin OprO of Pseudomonas aeruginosa: expression, purification and sequence Mol Microbiol. 1992 Aug;6(16):2319-26. doi: 10.1111/j.1365-2958.1992.tb01407.x. PMID: 1406271 | ||||
The bacterial porin superfamily: sequence alignment and structure prediction Mol Microbiol. 1991 Sep;5(9):2153-64. doi: 10.1111/j.1365-2958.1991.tb02145.x. PMID: 1662760 | ||||
| Proteins in this family with 3D-structure | ||||
View Entry | Description | Organism | Length | # strands |
