| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The Opacity porin protein Family [Function: Adhesion] Seed alignment | Full alignment | Pfam page | Pfam Wiki page | ||||
Members of this family are homologs to the surface protein A (NspA) from Neisseria meningitidis, which is a promising vaccine candidate. NspA is a highly conserved membrane protein that elicits protective antibody responses in mice against Neisseria meningitidis infection. NspA forms an 8-stranded antiparallel ß-barrel, whereas the four loops at the extracellular side of the NspA molecule form a long cleft, which contains mainly hydrophobic residues and harbors a detergent molecule, suggesting that the protein might function in the binding of hydrophobic ligands, such as lipids. Loops 2 and 3, are more important regarding antibody binding. | ||||
Representative image: 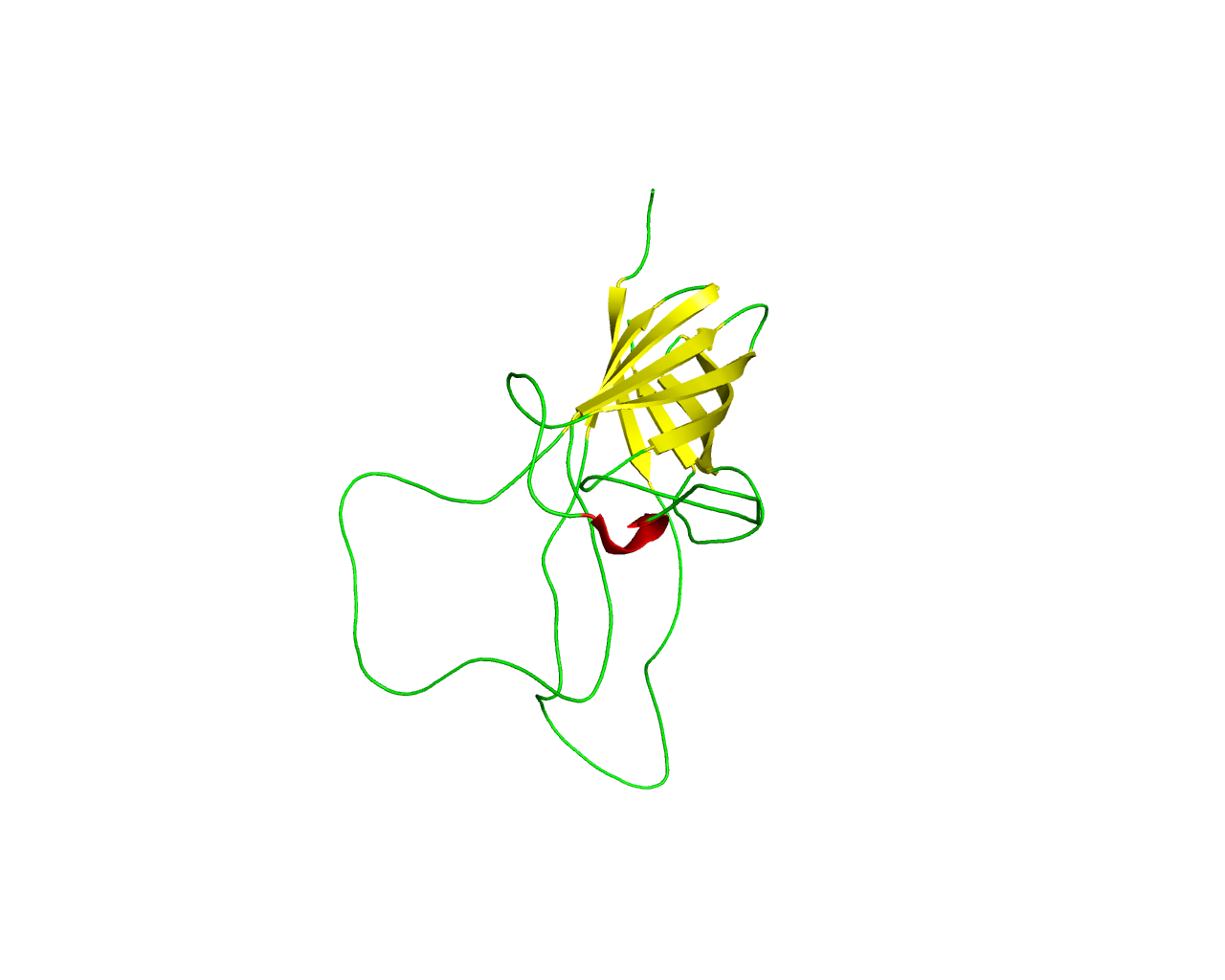 | ||||
| Literature references | ||||
A homopolymeric adenosine tract in the promoter region of nspA influences factor H-mediated serum resistance in Neisseria meningitidis Sci Rep. 2019 Feb 25;9(1):2736. doi: 10.1038/s41598-019-39231-0. PMID: 30804422 | ||||
Role of Gonococcal Neisserial Surface Protein A (NspA) in Serum Resistance and Comparison of Its Factor H Binding Properties with Those of Its Meningococcal Counterpart Infect Immun. 2019 Jan 24;87(2):e00658-18. doi: 10.1128/IAI.00658-18. Print 2019 Feb. PMID: 30510105 | ||||
Impaired Immunogenicity of Meningococcal Neisserial Surface Protein A in Human Complement Factor H Transgenic Mice Infect Immun. 2015 Nov 23;84(2):452-8. doi: 10.1128/IAI.01267-15. Print 2016 Feb. PMID: 26597984 | ||||
Binding of complement factor H to PorB3 and NspA enhances resistance of Neisseria meningitidis to anti-factor H binding protein bactericidal activity Infect Immun. 2015 Apr;83(4):1536-45. doi: 10.1128/IAI.02984-14. Epub 2015 Feb 2. PMID: 25644002 | ||||
Recombinant Neisseria surface protein A is a potential vaccine candidate against Neisseria meningitides serogroup B Mol Med Rep. 2014 Sep;10(3):1619-25. doi: 10.3892/mmr.2014.2325. Epub 2014 Jun 13. PMID: 24926810 | ||||
nspA gene as a specific genetic marker for detection of Neisseria meningitidis causing bacterial meningitis Indian J Biochem Biophys. 2014 Jun;51(3):211-4. PMID: 25204083 | ||||
fH-dependent complement evasion by disease-causing meningococcal strains with absent fHbp genes or frameshift mutations Vaccine. 2013 Aug 28;31(38):4192-9. doi: 10.1016/j.vaccine.2013.06.009. Epub 2013 Jun 17. PMID: 23791680 | ||||
[Cloning and expression of Neisserial surface protein A gene and its applicalion in detection of serum specific antibody] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2013 Apr;29(4):414-7. PMID: 23643173 | ||||
The relative roles of factor H binding protein, neisserial surface protein A, and lipooligosaccharide sialylation in regulation of the alternative pathway of complement on meningococci J Immunol. 2012 May 15;188(10):5063-72. doi: 10.4049/jimmunol.1103748. Epub 2012 Apr 13. PMID: 22504643 | ||||
Enhanced bacteremia in human factor H transgenic rats infected by Neisseria meningitidis Infect Immun. 2012 Feb;80(2):643-50. doi: 10.1128/IAI.05604-11. Epub 2011 Nov 21. PMID: 22104107 | ||||
Neisseria gonorrhoeae NspA induces specific bactericidal and opsonic antibodies in mice Clin Vaccine Immunol. 2011 Nov;18(11):1817-22. doi: 10.1128/CVI.05245-11. Epub 2011 Sep 14. PMID: 21918113 | ||||
Meningococcal group W-135 and Y capsular polysaccharides paradoxically enhance activation of the alternative pathway of complement J Biol Chem. 2011 Mar 11;286(10):8297-307. doi: 10.1074/jbc.M110.184838. Epub 2011 Jan 18. PMID: 21245150 | ||||
Conformational epitopes recognized by protective anti-neisserial surface protein A antibodies Infect Immun. 2003 Dec;71(12):6844-9. doi: 10.1128/iai.71.12.6844-6849.2003. PMID: 14638771 | ||||
Crystal structure of Neisserial surface protein A (NspA), a conserved outer membrane protein with vaccine potential J Biol Chem. 2003 Jul 4;278(27):24825-30. doi: 10.1074/jbc.M302803200. Epub 2003 Apr 26. PMID: 12716881 | ||||
The role of neisserial Opa proteins in interactions with host cells Trends Microbiol. 1998 Dec;6(12):489-95. doi: 10.1016/s0966-842x(98)01365-1. PMID: 10036728 | ||||
Highly conserved Neisseria meningitidis surface protein confers protection against experimental infection J Exp Med. 1997 Apr 7;185(7):1173-83. doi: 10.1084/jem.185.7.1173. PMID: 9104804 | ||||
| Proteins in this family with 3D-structure | ||||
View Entry | Description | Organism | Length | # strands |
