| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The Omptin Family [Function: Enzyme] Seed alignment | Full alignment | Pfam page | Pfam Wiki page | TC-DB page | ||||
Omptins are a family of enterobacterial surface proteases/adhesins that share high sequence identity and a conserved ß-barrel fold in the outer membrane. The omptins are multifunctional, and the individual omptins exhibit differing virulence-associated functions.This family contains a number of integral outer membrane serine proteases that differ from trypsin-like proteases in that they cleave polypeptides between two basically charged amino acids. Escherichia coli OmpT protein has previously been classified as a serine protease with Ser(99) and His(212) as active site residues. The X-ray structure of the Enzyme is inconsistent with this classification, and the involvement of a nucleophilic water molecule, activated by the residue pair Asp(210)/His(212), classifies the enzyme as an aspartic endopeptidase with the activity also strongly dependent on Asp(83) and Asp(85). Both may function in binding of the water molecule and/or in oxyanion stabilization. The transmembrane ß-barrel, consists of 10 ß-strands, and the enzymatic activity depends on reversible dimerization. | ||||
Representative image: 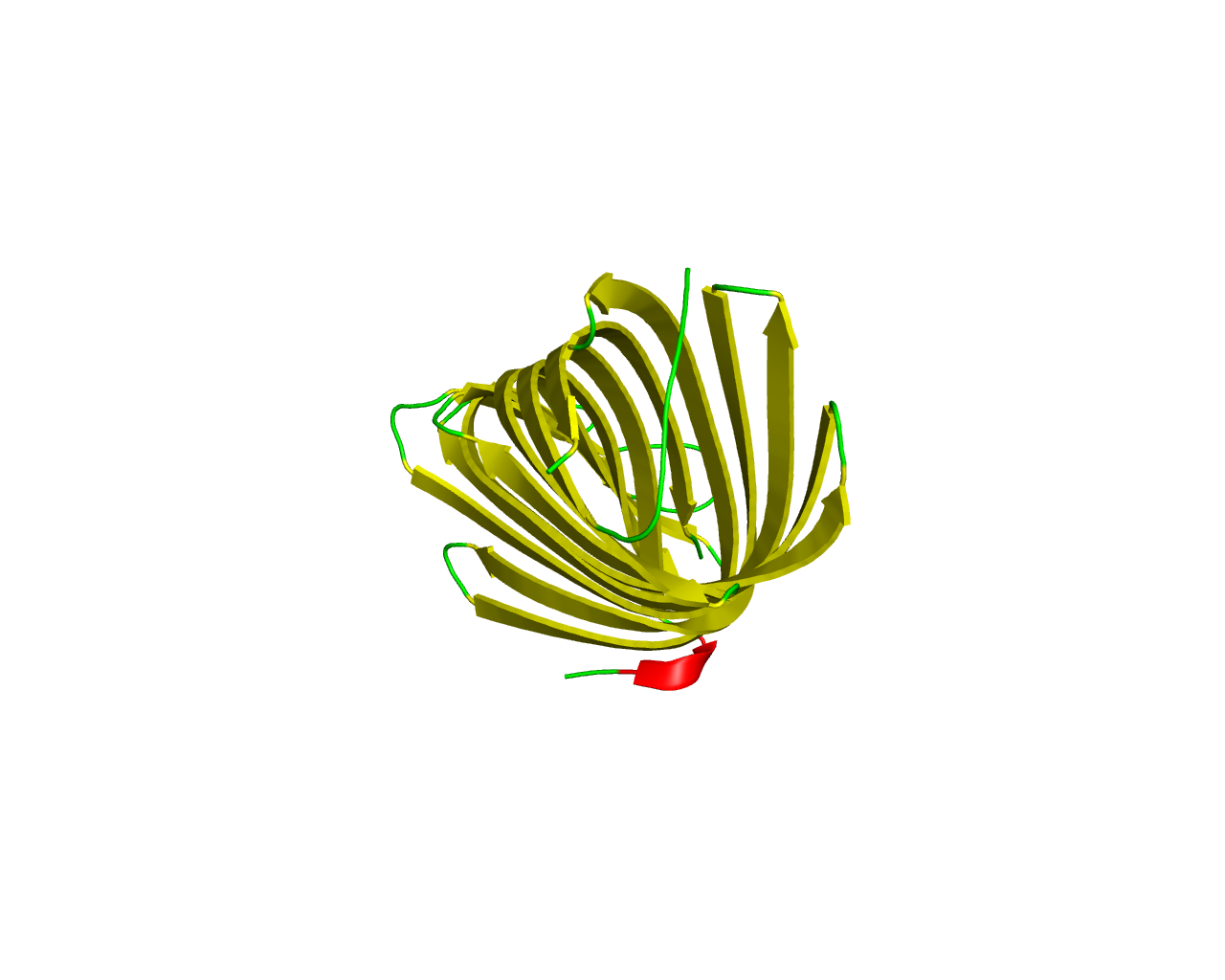 | ||||
| Literature references | ||||
Inhibition of outer membrane proteases of the omptin family by aprotinin Infect Immun. 2015 Jun;83(6):2300-11. doi: 10.1128/IAI.00136-15. Epub 2015 Mar 30. PMID: 25824836 | ||||
Fibrinolytic and coagulative activities of Yersinia pestis Front Cell Infect Microbiol. 2013 Jul 26;3:35. doi: 10.3389/fcimb.2013.00035. eCollection 2013. PMID: 23898467 | ||||
Molecular adaptation of a plant-bacterium outer membrane protease towards plague virulence factor Pla BMC Evol Biol. 2011 Feb 11;11:43. doi: 10.1186/1471-2148-11-43. PMID: 21310089 | ||||
An active site water network in the plasminogen activator pla from Yersinia pestis Structure. 2010 Jul 14;18(7):809-18. doi: 10.1016/j.str.2010.03.013. PMID: 20637417 | ||||
Omptin proteins: an expanding family of outer membrane proteases in Gram-negative Enterobacteriaceae Mol Membr Biol. 2007 Sep-Dec;24(5-6):395-406. doi: 10.1080/09687680701443822. PMID: 17710644 | ||||
Structural biology of membrane-intrinsic beta-barrel enzymes: sentinels of the bacterial outer membrane Biochim Biophys Acta. 2008 Sep;1778(9):1881-96. doi: 10.1016/j.bbamem.2007.07.021. Epub 2007 Aug 11. PMID: 17880914 | ||||
Substrate specificity of the Escherichia coli outer membrane protease OmpT J Bacteriol. 2004 Sep;186(17):5919-25. doi: 10.1128/JB.186.17.5919-5925.2004. PMID: 15317797 | ||||
The omptin family of enterobacterial surface proteases/adhesins: from housekeeping in Escherichia coli to systemic spread of Yersinia pestis Int J Med Microbiol. 2004 Jul;294(1):7-14. doi: 10.1016/j.ijmm.2004.01.003. PMID: 15293449 | ||||
Identification of essential acidic residues of outer membrane protease OmpT supports a novel active site FEBS Lett. 2001 Sep 21;505(3):426-30. doi: 10.1016/s0014-5793(01)02863-0. PMID: 11576541 | ||||
Crystal structure of the outer membrane protease OmpT from Escherichia coli suggests a novel catalytic site EMBO J. 2001 Sep 17;20(18):5033-9. doi: 10.1093/emboj/20.18.5033. PMID: 11566868 | ||||
| Proteins in this family with 3D-structure | ||||
View Entry | Description | Organism | Length | # strands |
