| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The LPS transport system D (LptD) Family [Function: Biogenesis/Secretion] Seed alignment | Full alignment | Pfam page | TC-DB page | ||||
In Gram-negative bacteria, the components of the outer membrane are synthesized in the cytoplasm or the inner membrane and must therefore traverse the inner membrane and the periplasm on the way to their final destination. Lipopolysaccharide molecules (LPS) are an essential component of the bacterial outer membrane and consist of a hydrophobic membrane anchor (lipid A) and an oligosaccharide core region that can be extended in some bacteria by a repeating oligosaccharide, the O-antigen. An outer membrane protein, which probably forms a ß-barrel, is required for the appearance of LPS at the bacterial cell surface. This protein is known as Imp (increased membrane permeability) or OstA (organic solvent tolerance) because Escherichia coli strains expressing mutant versions of this protein showed altered membrane permeability. LptD is an essential OMP that mediates the final transport of LPS to outer leaflet. LptD forms a novel 26-stranded ß-barrel, which is to our knowledge one of the largest ß-barrel reported so far. LptE adopts a roll-like structure located inside the barrel of LptD to form an unprecedented two-protein 'barrel and plug' architecture. The structure, molecular dynamics simulations and functional assays suggest that the hydrophilic O-antigen and the core oligosaccharide of the LPS may pass through the barrel and the lipid A of the LPS may be inserted into the outer leaflet of the outer membrane through a lateral opening between strands ß1 and ß26 of LptD. | ||||
Representative image: 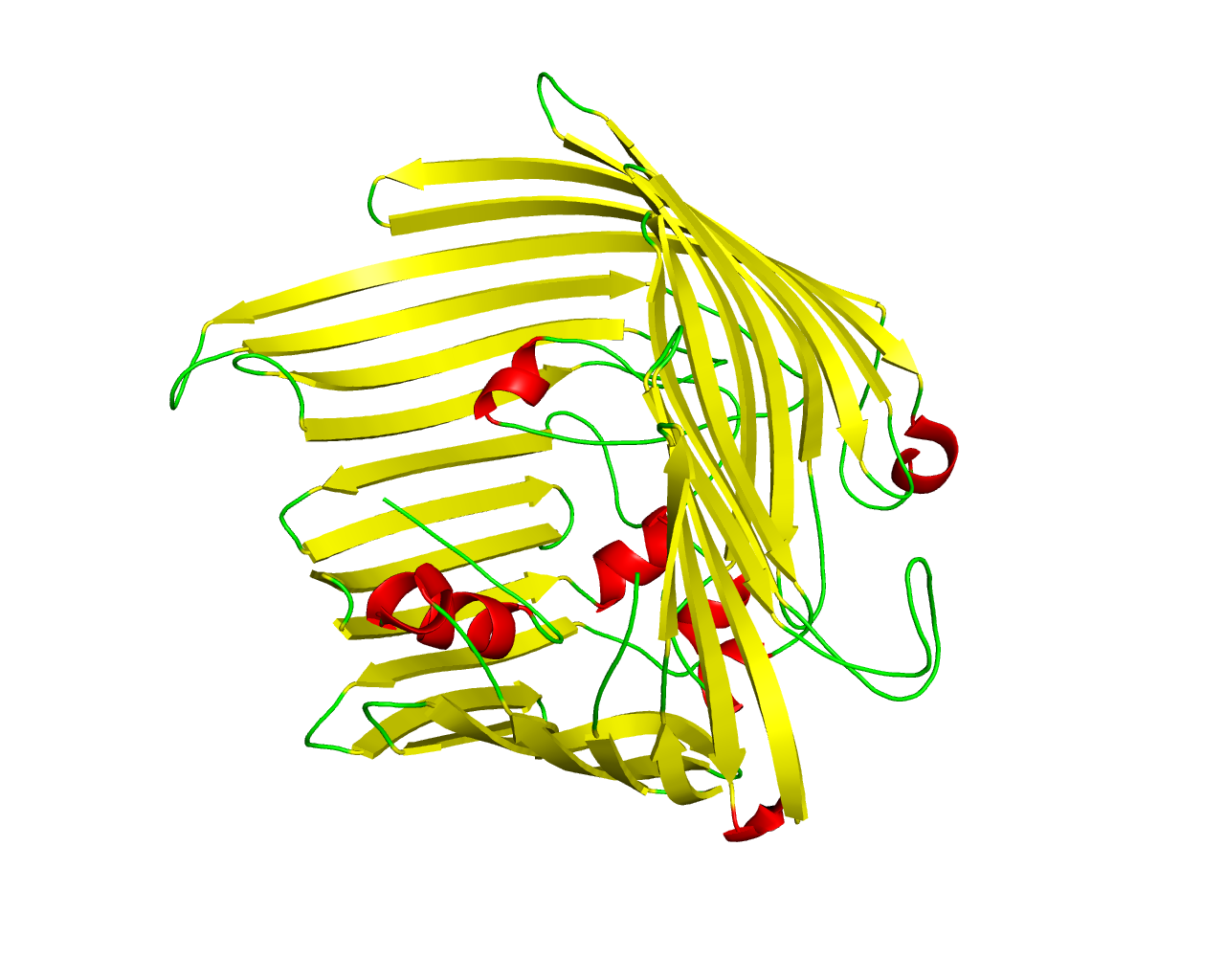 | ||||
| Literature references | ||||
Dynamics of an LPS translocon induced by substrate and an antimicrobial peptide Nat Chem Biol. 2021 Feb;17(2):187-195. doi: 10.1038/s41589-020-00694-2. Epub 2020 Nov 16. PMID: 33199913 | ||||
Reversible autoinhibitory regulation of Escherichia coli metallopeptidase BepA for selective ß-barrel protein degradation Proc Natl Acad Sci U S A. 2020 Nov 10;117(45):27989-27996. doi: 10.1073/pnas.2010301117. Epub 2020 Oct 22. PMID: 33093205 | ||||
Presence of substrate aids lateral gate separation in LptD Biochim Biophys Acta Biomembr. 2020 Jan 1;1862(1):183025. doi: 10.1016/j.bbamem.2019.07.013. Epub 2019 Jul 25. PMID: 31351059 | ||||
Substrate binding to BamD triggers a conformational change in BamA to control membrane insertion Proc Natl Acad Sci U S A. 2018 Mar 6;115(10):2359-2364. doi: 10.1073/pnas.1711727115. Epub 2018 Feb 20. PMID: 29463713 | ||||
A Peptidomimetic Antibiotic Interacts with the Periplasmic Domain of LptD from Pseudomonas aeruginosa ACS Chem Biol. 2018 Mar 16;13(3):666-675. doi: 10.1021/acschembio.7b00822. Epub 2018 Jan 23. PMID: 29359918 | ||||
Distinctive Roles for Periplasmic Proteases in the Maintenance of Essential Outer Membrane Protein Assembly J Bacteriol. 2017 Sep 19;199(20):e00418-17. doi: 10.1128/JB.00418-17. Print 2017 Oct 15. PMID: 28784813 | ||||
Protein Epitope Mimetics: From New Antibiotics to Supramolecular Synthetic Vaccines Acc Chem Res. 2017 Jun 20;50(6):1323-1331. doi: 10.1021/acs.accounts.7b00129. Epub 2017 Jun 1. PMID: 28570824 | ||||
LptD is a promising vaccine antigen and potential immunotherapeutic target for protection against Vibrio species infection Sci Rep. 2016 Dec 6;6:38577. doi: 10.1038/srep38577. PMID: 27922123 | ||||
Characterization of a stalled complex on the ß-barrel assembly machine Proc Natl Acad Sci U S A. 2016 Aug 2;113(31):8717-22. doi: 10.1073/pnas.1604100113. Epub 2016 Jul 20. PMID: 27439868 | ||||
Solution Structure and Dynamics of LptE from Pseudomonas aeruginosa Biochemistry. 2016 May 31;55(21):2936-43. doi: 10.1021/acs.biochem.6b00313. Epub 2016 May 19. PMID: 27166502 | ||||
Structural and Functional Characterization of the LPS Transporter LptDE from Gram-Negative Pathogens Structure. 2016 Jun 7;24(6):965-976. doi: 10.1016/j.str.2016.03.026. Epub 2016 May 5. PMID: 27161977 | ||||
The composition of the global and feature specific cyanobacterial core-genomes Front Microbiol. 2015 Mar 19;6:219. doi: 10.3389/fmicb.2015.00219. eCollection 2015. PMID: 25852675 | ||||
Accumulation of phosphatidic acid increases vancomycin resistance in Escherichia coli J Bacteriol. 2014 Sep;196(18):3214-20. doi: 10.1128/JB.01876-14. Epub 2014 Jun 23. PMID: 24957626 | ||||
Structural basis for lipopolysaccharide insertion in the bacterial outer membrane Nature. 2014 Jul 3;511(7507):108-11. doi: 10.1038/nature13484. Epub 2014 Jun 18. PMID: 24990751 | ||||
Structural basis for outer membrane lipopolysaccharide insertion Nature. 2014 Jul 3;511(7507):52-6. doi: 10.1038/nature13464. Epub 2014 Jun 18. PMID: 24990744 | ||||
LptE binds to and alters the physical state of LPS to catalyze its assembly at the cell surface Proc Natl Acad Sci U S A. 2014 Jul 1;111(26):9467-72. doi: 10.1073/pnas.1402746111. Epub 2014 Jun 17. PMID: 24938785 | ||||
Protease homolog BepA (YfgC) promotes assembly and degradation of ß-barrel membrane proteins in Escherichia coli Proc Natl Acad Sci U S A. 2013 Sep 17;110(38):E3612-21. doi: 10.1073/pnas.1312012110. Epub 2013 Sep 3. PMID: 24003122 | ||||
Biogenesis of the Gram-negative bacterial outer membrane Curr Opin Microbiol. 2004 Dec;7(6):610-6. doi: 10.1016/j.mib.2004.10.011. PMID: 15556033 | ||||
Identification of an outer membrane protein required for the transport of lipopolysaccharide to the bacterial cell surface Proc Natl Acad Sci U S A. 2004 Jun 22;101(25):9417-22. doi: 10.1073/pnas.0402340101. Epub 2004 Jun 10. PMID: 15192148 | ||||
Imp/OstA is required for cell envelope biogenesis in Escherichia coli Mol Microbiol. 2002 Sep;45(5):1289-302. doi: 10.1046/j.1365-2958.2002.03091.x. PMID: 12207697 | ||||
| Proteins in this family with 3D-structure | ||||
View Entry | Description | Organism | Length | # strands |
