| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The Outer membrane protein transport protein (OMPP1/FadL/TodX) Family [Function: Specific diffusion channels] Seed alignment | Full alignment | Pfam page | Pfam Wiki page | TC-DB page | ||||
This family includes several distantly related outer membrane proteins, sequenced from different bacterial species. The family is named after the Escherichia coli FadL protein which functions in transporting long chain fatty acids across the outer membrane. Other members (such as XylN and TodX) are implicated in the transport of aromatic compounds such as toluene, m-xylene and its analogs. All members seem to function as monomers. The 3D structure of FadL at high resolution shows that the protein forms a monomeric ß-barrel composed of 14 ß strands, that is occluded by a central hatch domain. The hatch domain is composed of residues in the N-terminus in a similar manner with that found in the TonB-dependent receptors. The hydrophobic compounds probably bind to multiple sites in the channel and use a transport mechanism that involves a spontaneous conformational change in the hatch region. | ||||
Representative image: 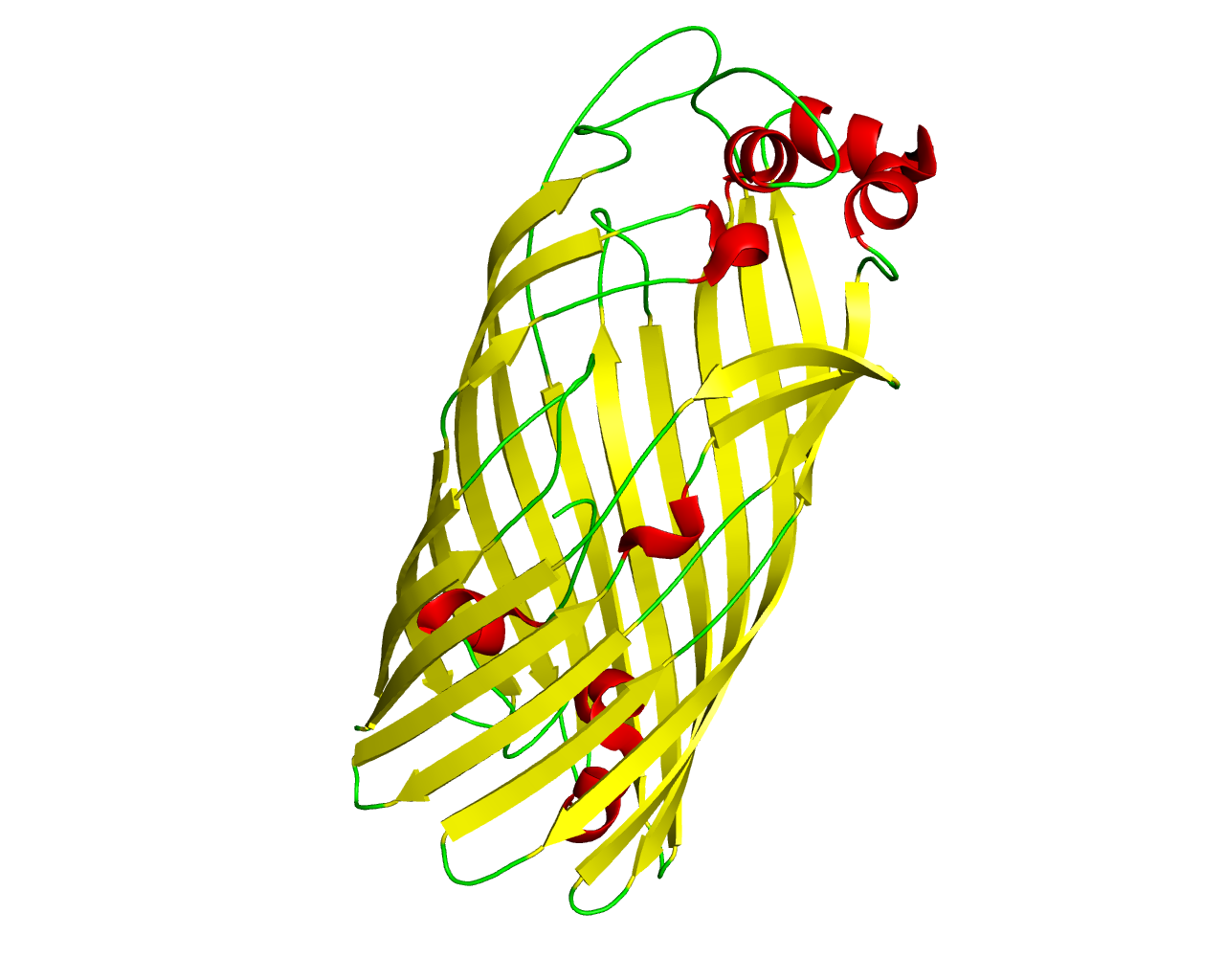 | ||||
| Literature references | ||||
Novel Bacterial Surface Display System Based on the Escherichia coli Protein MipA J Microbiol Biotechnol. 2020 Jul 28;30(7):1097-1103. doi: 10.4014/jmb.2001.01053. PMID: 32325544 | ||||
Antagonistic Pleiotropy in the Bifunctional Surface Protein FadL (OmpP1) during Adaptation of Haemophilus influenzae to Chronic Lung Infection Associated with Chronic Obstructive Pulmonary Disease mBio. 2018 Sep 25;9(5):e01176-18. doi: 10.1128/mBio.01176-18. PMID: 30254117 | ||||
Production of (Z)-11-(heptanoyloxy)undec-9-enoic acid from ricinoleic acid by utilizing crude glycerol as sole carbon source in engineered Escherichia coli expressing BVMO-ADH-FadL Enzyme Microb Technol. 2018 Dec;119:45-51. doi: 10.1016/j.enzmictec.2018.09.001. Epub 2018 Sep 5. PMID: 30243386 | ||||
Intracellular transformation rates of fatty acids are influenced by expression of the fatty acid transporter FadL in Escherichia coli cell membrane J Biotechnol. 2018 Sep 10;281:161-167. doi: 10.1016/j.jbiotec.2018.07.019. Epub 2018 Jul 21. PMID: 30016739 | ||||
Identification of ethanol tolerant outer membrane proteome reveals OmpC-dependent mechanism in a manner of EnvZ/OmpR regulation in Escherichia coli J Proteomics. 2018 May 15;179:92-99. doi: 10.1016/j.jprot.2018.03.005. Epub 2018 Mar 6. PMID: 29518576 | ||||
Improving Escherichia coli membrane integrity and fatty acid production by expression tuning of FadL and OmpF Microb Cell Fact. 2017 Feb 28;16(1):38. doi: 10.1186/s12934-017-0650-8. PMID: 28245829 | ||||
Molecular variation of the nonribosomal peptide-polyketide siderophore yersiniabactin through biosynthetic and metabolic engineering Biotechnol Bioeng. 2016 May;113(5):1067-74. doi: 10.1002/bit.25872. Epub 2015 Nov 20. PMID: 26524346 | ||||
Outer membrane proteomics of kanamycin-resistant Escherichia coli identified MipA as a novel antibiotic resistance-related protein FEMS Microbiol Lett. 2015 Jun;362(11):fnv074. doi: 10.1093/femsle/fnv074. Epub 2015 May 3. PMID: 25940639 | ||||
Substrate uptake and subcellular compartmentation of anoxic cholesterol catabolism in Sterolibacterium denitrificans J Biol Chem. 2015 Jan 9;290(2):1155-69. doi: 10.1074/jbc.M114.603779. Epub 2014 Nov 21. PMID: 25418128 | ||||
Identification of membrane proteins associated with phenylpropanoid tolerance and transport in Escherichia coli BL21 J Proteomics. 2015 Jan 15;113:15-28. doi: 10.1016/j.jprot.2014.09.012. Epub 2014 Sep 30. PMID: 25277045 | ||||
fadD deletion and fadL overexpression in Escherichia coli increase hydroxy long-chain fatty acid productivity Appl Microbiol Biotechnol. 2014 Nov;98(21):8917-25. doi: 10.1007/s00253-014-5974-2. Epub 2014 Aug 14. PMID: 25117545 | ||||
Rhizobial homologs of the fatty acid transporter FadL facilitate perception of long-chain acyl-homoserine lactone signals Proc Natl Acad Sci U S A. 2014 Jul 22;111(29):10702-7. doi: 10.1073/pnas.1404929111. Epub 2014 Jul 7. PMID: 25002473 | ||||
The outer surface lipoprotein VolA mediates utilization of exogenous lipids by Vibrio cholerae mBio. 2013 May 14;4(3):e00305-13. doi: 10.1128/mBio.00305-13. PMID: 23674613 | ||||
Functional characterization of ExFadLO, an outer membrane protein required for exporting oxygenated long-chain fatty acids in Pseudomonas aeruginosa Biochimie. 2013 Feb;95(2):290-8. doi: 10.1016/j.biochi.2012.09.032. Epub 2012 Oct 12. PMID: 23069386 | ||||
Crosstalk of Escherichia coli FadR with global regulators in expression of fatty acid transport genes PLoS One. 2012;7(9):e46275. doi: 10.1371/journal.pone.0046275. Epub 2012 Sep 28. PMID: 23029459 | ||||
Screening of the Salmonella paratyphi A CMCC 50973 strain outer membrane proteins for the identification of potential vaccine targets Mol Med Rep. 2012 Jan;5(1):78-83. doi: 10.3892/mmr.2011.587. Epub 2011 Sep 13. PMID: 21922141 | ||||
Outer membrane proteome and its regulation networks in response to glucose concentration changes in Escherichia coli Mol Biosyst. 2011 Nov;7(11):3087-93. doi: 10.1039/c1mb05193h. Epub 2011 Aug 18. PMID: 21850335 | ||||
Ligand-gated diffusion across the bacterial outer membrane Proc Natl Acad Sci U S A. 2011 Jun 21;108(25):10121-6. doi: 10.1073/pnas.1018532108. Epub 2011 May 18. PMID: 21593406 | ||||
Transmembrane passage of hydrophobic compounds through a protein channel wall Nature. 2009 Mar 19;458(7236):367-70. doi: 10.1038/nature07678. Epub 2009 Feb 1. PMID: 19182779 | ||||
Outer-membrane transport of aromatic hydrocarbons as a first step in biodegradation Proc Natl Acad Sci U S A. 2008 Jun 24;105(25):8601-6. doi: 10.1073/pnas.0801264105. Epub 2008 Jun 16. PMID: 18559855 | ||||
The FadL family: unusual transporters for unusual substrates Curr Opin Struct Biol. 2005 Aug;15(4):401-7. doi: 10.1016/j.sbi.2005.06.003. PMID: 16005205 | ||||
Bacterial long chain fatty acid transport: gateway to a fatty acid-responsive signaling system J Biol Chem. 2004 Nov 26;279(48):49563-6. doi: 10.1074/jbc.R400026200. Epub 2004 Aug 30. PMID: 15347640 | ||||
Crystal structure of the long-chain fatty acid transporter FadL Science. 2004 Jun 4;304(5676):1506-9. doi: 10.1126/science.1097524. PMID: 15178802 | ||||
The TOL plasmid pWW0 xylN gene product from Pseudomonas putida is involved in m-xylene uptake J Bacteriol. 2001 Nov;183(22):6662-6. doi: 10.1128/JB.183.22.6662-6666.2001. PMID: 11673437 | ||||
Identification of a membrane protein and a truncated LysR-type regulator associated with the toluene degradation pathway in Pseudomonas putida F1 Mol Gen Genet. 1995 Mar 10;246(5):570-9. doi: 10.1007/BF00298963. PMID: 7535376 | ||||
| Proteins in this family with 3D-structure | ||||
View Entry | Description | Organism | Length | # strands |
