| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The Aerolysin Family [Function: Non-specific diffusion channels] Seed alignment | Full alignment | Pfam page | TC-DB page | ||||
Aerolysin is a heptamer where seven monomers form a ß-barrel with 14 strands at the outer membrane of Aeromonas hydrophila. The aerolysin pore is more stable due its concentric ß-barrel fold. | ||||
Representative image: 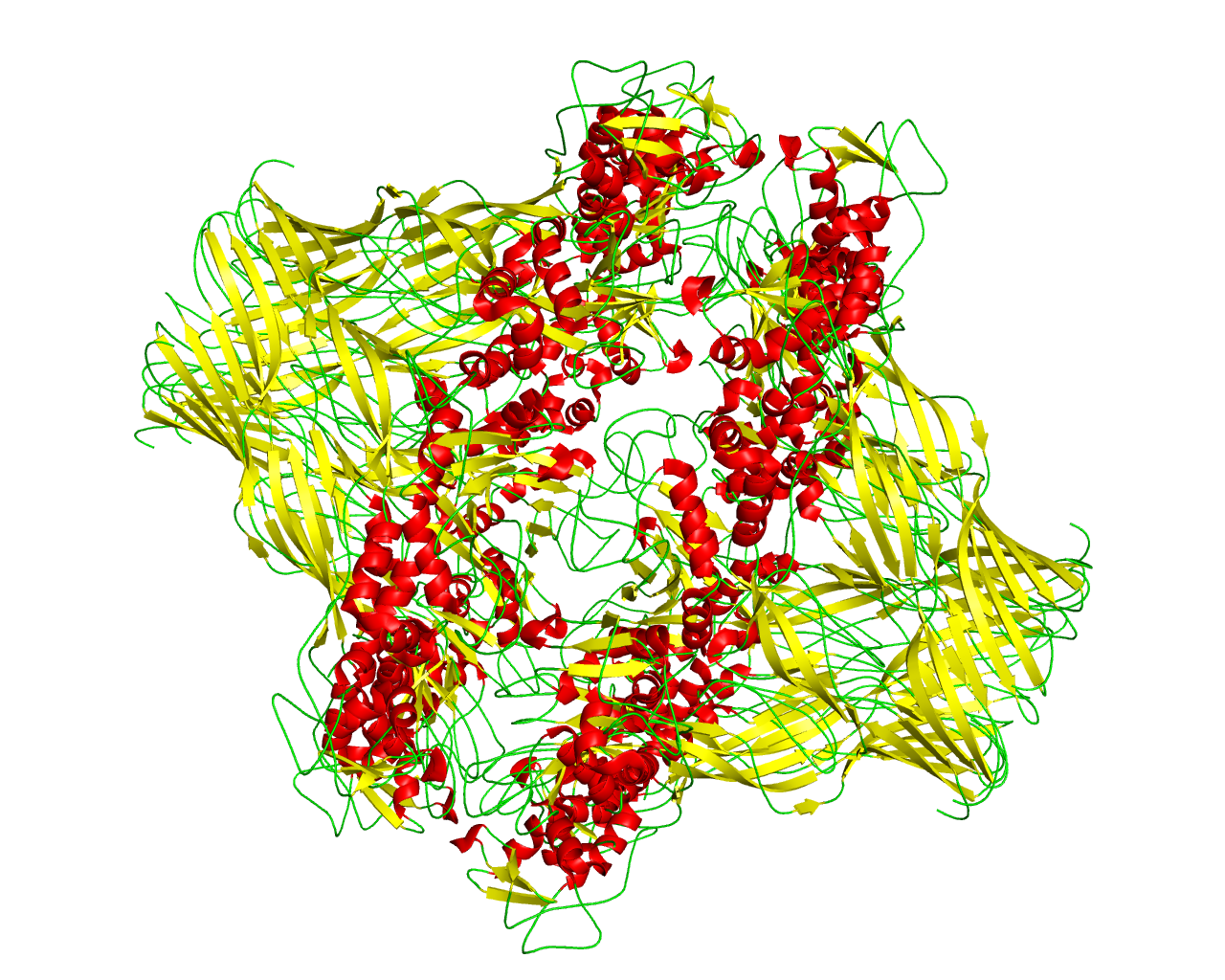 | ||||
| Literature references | ||||
Central residues of the amphipathic ß-hairpin loop control the properties of Clostridium perfringens epsilon-toxin channel Biochim Biophys Acta Biomembr. 2020 Sep 1;1862(9):183364. doi: 10.1016/j.bbamem.2020.183364. Epub 2020 May 22. PMID: 32450142 | ||||
Single-molecule sensing of peptides and nucleic acids by engineered aerolysin nanopores Nat Commun. 2019 Oct 29;10(1):4918. doi: 10.1038/s41467-019-12690-9. PMID: 31664022 | ||||
Insight into the Structural Dynamics of the Lysenin During Prepore-to-Pore Transition Using Hydrogen-Deuterium Exchange Mass Spectrometry Toxins (Basel). 2019 Aug 7;11(8):462. doi: 10.3390/toxins11080462. PMID: 31394843 | ||||
The pore structure of Clostridium perfringens epsilon toxin Nat Commun. 2019 Jun 14;10(1):2641. doi: 10.1038/s41467-019-10645-8. PMID: 31201325 | ||||
Enterotoxic Clostridia: Clostridium perfringens Enteric Diseases Microbiol Spectr. 2018 Sep;6(5):10.1128/microbiolspec.GPP3-0003-2017. doi: 10.1128/microbiolspec.GPP3-0003-2017. PMID: 30238869 | ||||
Rationally Designed Sensing Selectivity and Sensitivity of an Aerolysin Nanopore via Site-Directed Mutagenesis ACS Sens. 2018 Apr 27;3(4):779-783. doi: 10.1021/acssensors.8b00021. Epub 2018 Apr 17. PMID: 29619834 | ||||
Structural, physicochemical and dynamic features conserved within the aerolysin pore-forming toxin family Sci Rep. 2017 Oct 24;7(1):13932. doi: 10.1038/s41598-017-13714-4. PMID: 29066778 | ||||
Cryo-EM structure of aerolysin variants reveals a novel protein fold and the pore-formation process Nat Commun. 2016 Jul 13;7:12062. doi: 10.1038/ncomms12062. PMID: 27405240 | ||||
Crystal structure of an invertebrate cytolysin pore reveals unique properties and mechanism of assembly Nat Commun. 2016 May 12;7:11598. doi: 10.1038/ncomms11598. PMID: 27176125 | ||||
Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein Nat Commun. 2016 Apr 6;7:11293. doi: 10.1038/ncomms11293. PMID: 27048994 | ||||
Dynamics and Energy Contributions for Transport of Unfolded Pertactin through a Protein Nanopore ACS Nano. 2015 Sep 22;9(9):9050-61. doi: 10.1021/acsnano.5b03053. Epub 2015 Aug 28. PMID: 26302243 | ||||
Molecular assembly of the aerolysin pore reveals a swirling membrane-insertion mechanism Nat Chem Biol. 2013 Oct;9(10):623-9. doi: 10.1038/nchembio.1312. Epub 2013 Aug 4. PMID: 23912165 | ||||
Epsilon toxin: a fascinating pore-forming toxin FEBS J. 2011 Dec;278(23):4602-15. doi: 10.1111/j.1742-4658.2011.08145.x. Epub 2011 May 25. PMID: 21535407 | ||||
Pore-forming activity of alpha-toxin is essential for clostridium septicum-mediated myonecrosis Infect Immun. 2009 Mar;77(3):943-51. doi: 10.1128/IAI.01267-08. Epub 2009 Jan 12. PMID: 19139192 | ||||
A rivet model for channel formation by aerolysin-like pore-forming toxins EMBO J. 2006 Feb 8;25(3):457-66. doi: 10.1038/sj.emboj.7600959. Epub 2006 Jan 19. PMID: 16424900 | ||||
| Proteins in this family with 3D-structure | ||||
View Entry | Description | Organism | Length | # strands |
