| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The Outer membrane usher protein Family [Function: Biogenesis/Secretion] Seed alignment | Full alignment | Pfam page | Pfam Wiki page | TC-DB page | ||||
Ushers are a family of integral outer membrane proteins participating in the chaperone (usher) secretion pathway, a terminal branch of the General Secretion Pathway (GSP) dedicated to the biogenesis of adhesive surface structures associated with pathogenesis. As the name of the pathway implies, these proteins work in conjunction with a periplasmic chaperone in order to assemble and secrete more than 30 different surface molecules in a broad range of Gram-negative bacteria. The most studied example of the chaperone (usher) pathway is the assembly and biogenesis of the type 1 and P pili, expressed by the uropathogenic Escherichia coli. Members of the Usher family (like PapC, FasD, FaeD and FimD), display properties of ß-barrel proteins and form channels consisting of 24 ß-strands, as shown in the crystal structure of PapC. It has been proposed that Ushers form twin dimeric pores in the outer membrane and the C-terminal parts of the sequences are responsible for the dimerization. In addition, the N-terminal 100-120 amino acids are believed to be involved in the recognition of the periplasmic chaperone. | ||||
Representative image: 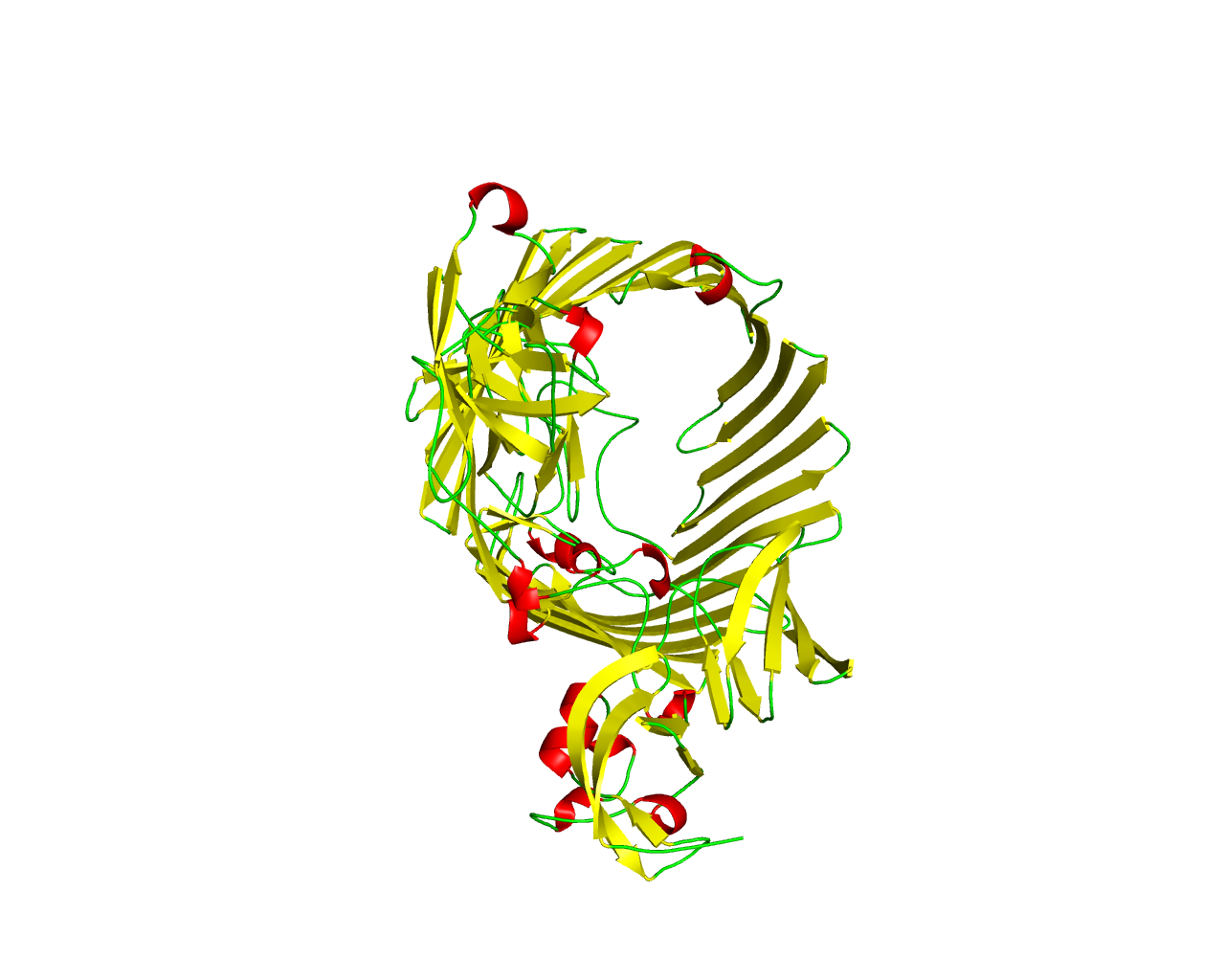 | ||||
| Literature references | ||||
Structural basis for usher activation and intramolecular subunit transfer in P pilus biogenesis in Escherichia coli Nat Microbiol. 2018 Dec;3(12):1362-1368. doi: 10.1038/s41564-018-0255-y. Epub 2018 Oct 1. PMID: 30275511 | ||||
Structural basis for substrate selection by the translocation and assembly module of the ß-barrel assembly machinery Mol Microbiol. 2017 Oct;106(1):142-156. doi: 10.1111/mmi.13757. Epub 2017 Aug 9. PMID: 28752534 | ||||
Effect of chaperone-adhesin complex on plug release by the PapC usher FEBS Lett. 2016 Jul;590(14):2172-9. doi: 10.1002/1873-3468.12257. Epub 2016 Jul 4. PMID: 27313078 | ||||
Electrostatic networks control plug stabilization in the PapC usher Mol Membr Biol. 2015 Aug-Dec;32(5-8):198-207. doi: 10.3109/09687688.2016.1160450. Epub 2016 May 16. PMID: 27181766 | ||||
Lifting the lid on pilus assembly Elife. 2014 Oct 28;3:e04997. doi: 10.7554/eLife.04997. PMID: 25350974 | ||||
Allosteric signalling in the outer membrane translocation domain of PapC usher Elife. 2014 Oct 28;3:e03532. doi: 10.7554/eLife.03532. PMID: 25271373 | ||||
Molecular basis of usher pore gating in Escherichia coli pilus biogenesis Proc Natl Acad Sci U S A. 2013 Dec 17;110(51):20741-6. doi: 10.1073/pnas.1320528110. Epub 2013 Dec 2. PMID: 24297893 | ||||
Domain activities of PapC usher reveal the mechanism of action of an Escherichia coli molecular machine Proc Natl Acad Sci U S A. 2012 Jun 12;109(24):9563-8. doi: 10.1073/pnas.1207085109. Epub 2012 May 29. PMID: 22645361 | ||||
The role of chaperone-subunit usher domain interactions in the mechanism of bacterial pilus biogenesis revealed by ESI-MS Mol Cell Proteomics. 2012 Jul;11(7):M111.015289. doi: 10.1074/mcp.M111.015289. Epub 2012 Feb 27. PMID: 22371487 | ||||
The fimbrial usher FimD follows the SurA-BamB pathway for its assembly in the outer membrane of Escherichia coli J Bacteriol. 2011 Oct;193(19):5222-30. doi: 10.1128/JB.05585-11. Epub 2011 Jul 22. PMID: 21784935 | ||||
Crystal structure of the FimD usher bound to its cognate FimC-FimH substrate Nature. 2011 Jun 2;474(7349):49-53. doi: 10.1038/nature10109. PMID: 21637253 | ||||
Structural homology between the C-terminal domain of the PapC usher and its plug J Bacteriol. 2010 Apr;192(7):1824-31. doi: 10.1128/JB.01677-09. Epub 2010 Jan 29. PMID: 20118254 | ||||
Insights into pilus assembly and secretion from the structure and functional characterization of usher PapC Proc Natl Acad Sci U S A. 2009 May 5;106(18):7403-7. doi: 10.1073/pnas.0902789106. Epub 2009 Apr 20. PMID: 19380723 | ||||
Fiber formation across the bacterial outer membrane by the chaperone/usher pathway Cell. 2008 May 16;133(4):640-52. doi: 10.1016/j.cell.2008.03.033. PMID: 18485872 | ||||
The outer membrane usher forms a twin-pore secretion complex J Mol Biol. 2004 Dec 10;344(5):1397-407. doi: 10.1016/j.jmb.2004.10.008. PMID: 15561151 | ||||
Topology of the outer membrane usher PapC determined by site-directed fluorescence labeling J Biol Chem. 2004 Dec 17;279(51):53747-54. doi: 10.1074/jbc.M409192200. Epub 2004 Oct 12. PMID: 15485883 | ||||
The usher N terminus is the initial targeting site for chaperone-subunit complexes and participates in subsequent pilus biogenesis events J Bacteriol. 2004 Aug;186(16):5321-31. doi: 10.1128/JB.186.16.5321-5331.2004. PMID: 15292133 | ||||
Ushers and secretins: channels for the secretion of folded proteins across the bacterial outer membrane J Mol Microbiol Biotechnol. 2002 Jan;4(1):11-20. PMID: 11763968 | ||||
| Proteins in this family with 3D-structure | ||||
View Entry | Description | Organism | Length | # strands |
