| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The Membrane Attack Complex/Perforin (MACPF) Family [Function: Non-specific diffusion channels] Seed alignment | Full alignment | Pfam page | TC-DB page | ||||
The membrane attack complex (MAC) is a structure formed at the outer membrane of pathogen cells resulting from activation of the host's complement system. It is one of the immune system's first responders. MAC forms damaging transmembrane channels at the cell membrane of pathogens causing cell lysis and death. It is a multiprotein complex, comprised of 6 polypeptide chains (C5b, C6, C7, C8α, C8&ß, and C8γ) together with multiple copies of C9 monomer that are arranged in a split-washer configuration, composing a ring in the membrane which permits free flux of molecules in and out of the cell. The cell dies when enough pores are form. Membrane Attack Complex-Perforin (MACPF) domain (PF01823) is a common domain in all C6-C9. MAC assembly into the membrane starts when C7 connects to C5b6 (a complex formed by C5b and C6) to compose MAC precursor C5b7. Subsequently, C8 connects irreversibly to the former complex forming the membrane-inserted C5b8. Then, C5b8 binds to 22 C9 monomers to form C5b9 and polymerizes to finish the MAC pore formation. At the end, the ß barrel pore is composed after transforming the helical bundles in the MACPF domains into transmembrane ß hairpins through an unknown mechanism. According to the cryo-EM structure, the formed barrel consists of 88 strands. | ||||
Representative image: 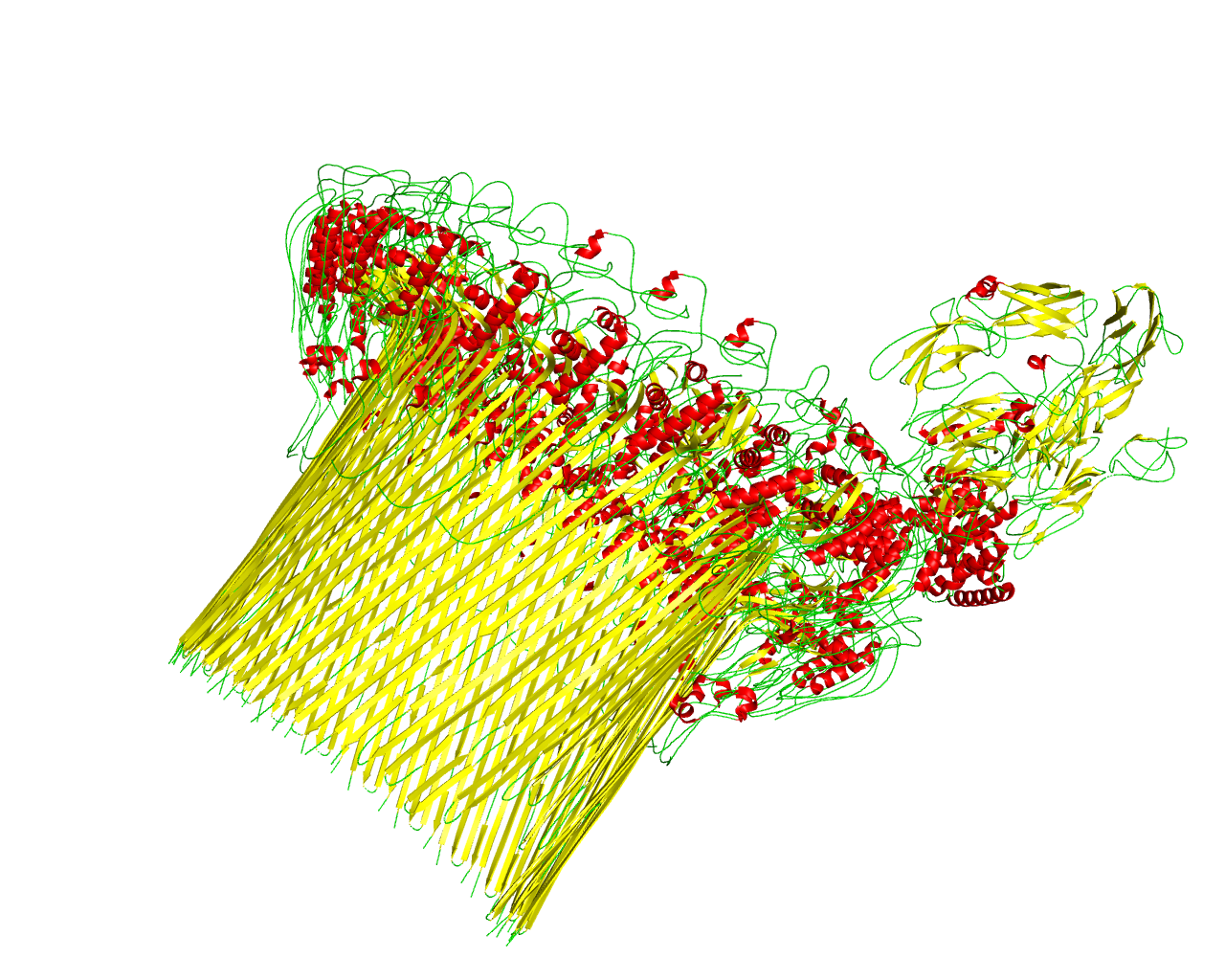 | ||||
| Literature references | ||||
An Intermolecular π-Stacking Interaction Drives Conformational Changes Necessary to ß-Barrel Formation in a Pore-Forming Toxin mBio. 2019 Jul 2;10(4):e01017-19. doi: 10.1128/mBio.01017-19. PMID: 31266869 | ||||
The Structural Basis for a Transition State That Regulates Pore Formation in a Bacterial Toxin mBio. 2019 Apr 23;10(2):e00538-19. doi: 10.1128/mBio.00538-19. PMID: 31015325 | ||||
Identification of a Fifth Antibacterial Toxin Produced by a Single Bacteroides fragilis Strain J Bacteriol. 2019 Mar 26;201(8):e00577-18. doi: 10.1128/JB.00577-18. Print 2019 Apr 15. PMID: 30692177 | ||||
CryoEM reveals how the complement membrane attack complex ruptures lipid bilayers Nat Commun. 2018 Dec 14;9(1):5316. doi: 10.1038/s41467-018-07653-5. PMID: 30552328 | ||||
The mystery behind membrane insertion: a review of the complement membrane attack complex Philos Trans R Soc Lond B Biol Sci. 2017 Aug 5;372(1726):20160221. doi: 10.1098/rstb.2016.0221. PMID: 28630159 | ||||
Heterogeneous MAC Initiator and Pore Structures in a Lipid Bilayer by Phase-Plate Cryo-electron Tomography Cell Rep. 2016 Apr 5;15(1):1-8. doi: 10.1016/j.celrep.2016.03.002. Epub 2016 Mar 24. PMID: 27052168 | ||||
Structure of the poly-C9 component of the complement membrane attack complex Nat Commun. 2016 Feb 4;7:10588. doi: 10.1038/ncomms10588. PMID: 26841934 | ||||
Structural basis of complement membrane attack complex formation Nat Commun. 2016 Feb 4;7:10587. doi: 10.1038/ncomms10587. PMID: 26841837 | ||||
Giant MACPF/CDC pore forming toxins: A class of their own Biochim Biophys Acta. 2016 Mar;1858(3):475-86. doi: 10.1016/j.bbamem.2015.11.017. Epub 2015 Nov 26. PMID: 26607011 | ||||
The Apicomplexan CDC/MACPF-like pore-forming proteins Curr Opin Microbiol. 2015 Aug;26:48-52. doi: 10.1016/j.mib.2015.05.001. Epub 2015 May 27. PMID: 26025132 | ||||
Conformational changes during pore formation by the perforin-related protein pleurotolysin PLoS Biol. 2015 Feb 5;13(2):e1002049. doi: 10.1371/journal.pbio.1002049. eCollection 2015 Feb. PMID: 25654333 | ||||
A common fold mediates vertebrate defense and bacterial attack Science. 2007 Sep 14;317(5844):1548-51. doi: 10.1126/science.1144706. Epub 2007 Aug 23. PMID: 17717151 | ||||
The mechanism of membrane insertion for a cholesterol-dependent cytolysin: a novel paradigm for pore-forming toxins Cell. 1999 Oct 29;99(3):293-9. doi: 10.1016/s0092-8674(00)81660-8. PMID: 10555145 | ||||
Identification of a membrane-spanning domain of the thiol-activated pore-forming toxin Clostridium perfringens perfringolysin O: an alpha-helical to beta-sheet transition identified by fluorescence spectroscopy Biochemistry. 1998 Oct 13;37(41):14563-74. doi: 10.1021/bi981452f. PMID: 9772185 | ||||
The structure of human complement component C7 and the C5b-7 complex J Biol Chem. 1988 Jan 5;263(1):549-60. PMID: 3335508 | ||||
Structural/functional similarity between proteins involved in complement- and cytotoxic T-lymphocyte-mediated cytolysis Nature. 1986 Aug 28-Sep 3;322(6082):831-4. doi: 10.1038/322831a0. PMID: 2427956 | ||||
Evidence of direct insertion of terminal complement proteins into cell membrane bilayers during cytolysis. Labeling by a photosensitive membrane probe reveals a major role for the eighth and ninth components J Biol Chem. 1983 Apr 10;258(7):4318-24. PMID: 6833260 | ||||
Molecular organization of C9 within the membrane attack complex of complement. Induction of circular C9 polymerization by the C5b-8 assembly J Exp Med. 1982 Jul 1;156(1):268-82. doi: 10.1084/jem.156.1.268. PMID: 6177822 | ||||

|

|

|

|
