| Browse | Text search | Protein search | Domain search | Download | User manual | Related links | Citation & Contact |
The Curli production assembly/transport component (CsgG) Family [Function: Biogenesis / Secretion] Seed alignment | Full alignment | Pfam page | ||||
In Escherichia coli Xuzhou21, curli subunits are produced and secreted into the outer membrane through the CsgG secretion channel. Curli are a unique group of functional amyloids, which are crucial for host cell adhesion, biofilm formation and colonization of inert surfaces. They are involved in harmful diseases in humans, since they share similar structural and biochemical characteristics with amyloid fibers. Curli subunits are secreted into the outer membrane through the CsgG secretion channel. The crystal structure of CsgG showed that it is a symmetric nonameric channel, composed of monomers each having four strands spanning the outer membrane. A 36-stranded ß barrel is formed from nine CsgG monomers. CsgG could perhaps reduce the biofilm formation by controlling Curli secretion and that is why it is studied as a putative antibiotics target. | ||||
Representative image: 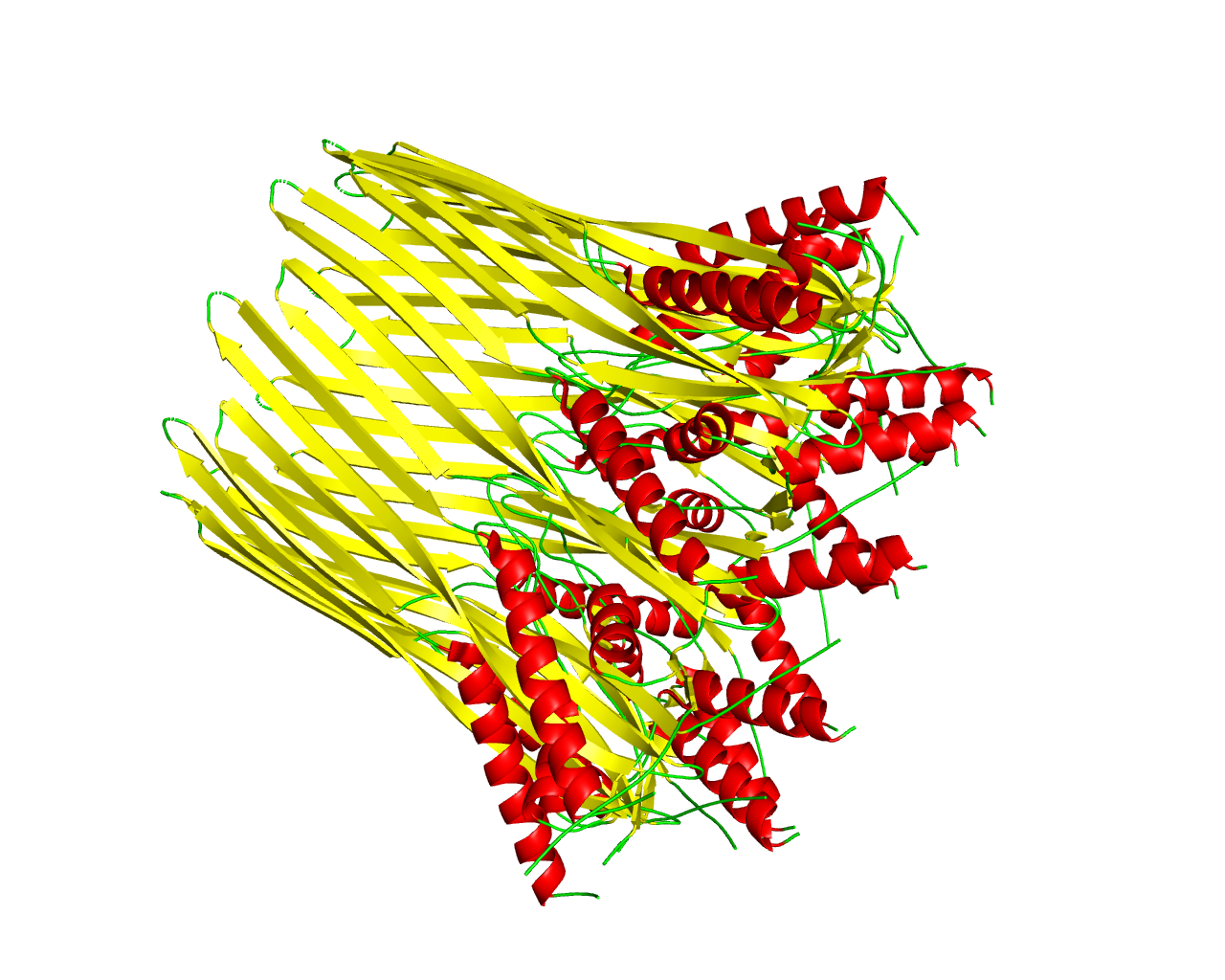 | ||||
| Literature references | ||||
Assembly of the secretion pores GspD, Wza and CsgG into bacterial outer membranes does not require the Omp85 proteins BamA or TamA Mol Microbiol. 2015 Aug;97(4):616-29. doi: 10.1111/mmi.13055. Epub 2015 Jun 6. PMID: 25976323 | ||||
Structure of the nonameric bacterial amyloid secretion channel Proc Natl Acad Sci U S A. 2014 Dec 16;111(50):E5439-44. doi: 10.1073/pnas.1411942111. Epub 2014 Dec 1. PMID: 25453093 | ||||
Structural and mechanistic insights into the bacterial amyloid secretion channel CsgG Nature. 2014 Dec 11;516(7530):250-3. doi: 10.1038/nature13768. Epub 2014 Sep 14. PMID: 25219853 | ||||
Misfolded protein aggregates: mechanisms, structures and potential for disease transmission Semin Cell Dev Biol. 2011 Jul;22(5):482-7. doi: 10.1016/j.semcdb.2011.04.002. Epub 2011 May 5. PMID: 21571086 | ||||
Curli biogenesis and function Annu Rev Microbiol. 2006;60:131-47. doi: 10.1146/annurev.micro.60.080805.142106. PMID: 16704339 | ||||
Common core structure of amyloid fibrils by synchrotron X-ray diffraction J Mol Biol. 1997 Oct 31;273(3):729-39. doi: 10.1006/jmbi.1997.1348. PMID: 9356260 | ||||

|

|

|

|
